
- Areas of Research
- Benefits of Research
- Research Studies
- Physician-Initiated Research
- Patient Stories
- Grand Rounds
- Clinical Research Internship
- Digital Apps for Physicians
- International Scholar Program
- Ways to Give
- Memorial & Tribute Giving
- Make a Planned Gift
- Become a Monthly Donor
- Articles & Newsletters
- Board of Directors

Clinical Research Internship Program
Developing and empowering the next generation of cardiovascular health professionals through a cutting-edge research internship program.
- Internship Application
- Meet The Interns
Educating the Next Generation
The Minneapolis Heart Institute Foundation® (MHIF) offers one of the most outstanding and unique internship opportunities available to undergraduates who are pre-med or planning a career in medicine. The goal of the research internship program is to develop and empower the next generation of cardiovascular health professionals by offering a robust research experience in the field of cardiology.
During this unique educational experience, the research intern is paired with a physician mentor who is actively engaged as a clinician and researcher at a nationally recognized hospital. At the conclusion of the program, the interns will showcase their research work at an open house and poster session. Many projects result in the intern having the opportunity to be an author on an article published in a national scientific journal. Some interns also have the opportunity to present their work at a national cardiovascular conference.
This internship is made possible thanks to philanthropic support from generous donors.

Research Internship Details
Research Internship Overview
Be a part of the Minneapolis Heart Institute Foundation’s exceptional 12-week cardiovascular clinical research internship designed for undergraduate students who are pre-med or planning a career in medicine. During this unique educational experience you will be paired with a physician mentor who is actively engaged as a clinician and researcher at a nationally recognized hospital. You will have the opportunity to:
- Participate in groundbreaking cardiovascular research
- Shadow a physician mentor in hospital rounds, watch procedures and listen as care teams discuss cases.
- Observe an open-heart procedure.
- Attend case conferences where cross-functional care teams discuss difficult cases.
- Shadow cardiovascular nurses in the ICU and post-surgical care area.
- Observe leading-edge cardiovascular diagnostic services.
- Reflect on your career options while hearing physicians’ candid stories of career journeys and passions during lunch and learns.
- Participate in interactive medical device industry tours and a hands-on tour of cardiovascular specimens at a nationally renowned heart registry.
At the conclusion of the internship, you will showcase your research work at an open house and poster session. Many projects result in the intern having the opportunity to be an author on an article published in a national scientific journal. Some interns will also have the opportunity to present their work at a national cardiovascular conference.
Location: On the campus of the flagship hospital of Allina Health, Abbott Northwestern Hospital, in Minneapolis, Minnesota.
Program dates: May 28-August 15, 2025
Compensation: A $550 per week stipend paid bi-weekly to assist with expenses for housing and living expenses (no direct housing provided). Scholarship opportunities may be available for accepted applicants who need further support for travel or living expenses.
Application Opens: November 8, 2024
Deadline: January 3, 2025
Selection Notification: March 10, 2025
Program Dates: May 28-August 15, 2025
Eligibility
- Undergraduate or recent graduate from a four-year college and not yet accepted to medical school. Preference will be given to those who have completed their junior year by the start of the research internship. Strong applicants who have completed their sophomore year or just graduated from college may also be considered.
- Must be enrolled in or a very recent graduate from an accredited degree program and not yet accepted to medical school.
- GPA of 3.60 or above.
- Applicants must be US citizens or have Permanent Resident status (be able to provide proof of employability through completion of the I-9 Form).
- The program welcomes applicants from all backgrounds, including but not limited to BIPOC (Black, Indigenous, People of Color) individuals, individuals who are historically underrepresented in health-related sciences and medical fields, individuals who face greater obstacles to educational achievements and individuals who can contribute to diversity, equity and inclusion efforts in the health-related sciences and medical fields.
- Must be able to complete 8-12 hours of independent, online CITI training (basic training required to participate in human subjects/clinical research) by April 30.
- Available to participate full-time (at least 40 hours per week) in the internship May 28 through August 15, 2025
Steps Before You Apply
- Review the sample application PDF and prepare responses to the short answer and essay questions. Please note: this is an example and the application for the next cohort may change.
- Write a personal motivation statement (3,500 characters including spaces).
- Prepare a one-page resume (save as PDF or Word doc with file name First Name_Last Name).
- Note that you will have the opportunity to make changes to your application before submitting.
Program Components
In preparing for a career in medicine, this experience will position you well through a unique opportunity to participate in cardiovascular clinical research, shadow clinicians in action and witness everything from a routine day to extraordinary life-saving procedures.
- Orientation: Three days of orientation will prepare you for your research project, ground you in cardiovascular essentials and introduce you to the colleagues you will be working with, including your research physician, staff mentor and fellow interns.
- Research project: You will be paired with a physician who is the Principal Investigator on a research project and a staff mentor. In addition to capturing data from the electronic health record, organizing and analyzing the data and preparing conclusions, you may also write case reports and support other research projects.
- Observations: To provide you with exposure to what a career is really like in the field of medicine, you will observe various cardiovascular procedures, shadow healthcare professionals and visit cardiovascular related departments at Abbott Northwestern Hospital. Additionally, interns shadow healthcare professionals in the ICU and post-procedural care areas.
- Field Trips: Minneapolis is a hub for headquarters of major medical device companies and start-ups. You will have custom tours of some major medical device companies, the renowned Visible Heart Lab at the University of Minnesota and the Jesse E. Edwards Registry of Cardiovascular Disease. This registry is a repository for heart specimens of almost every known type of heart disease, including rare congenital and adult heart diseases.
- Poster Session: The culminating event of the research internship program, you will display your outcomes on a research poster and present to mentors, other medical professionals, MHIF board members, family and friends.
- Cohort Activities: The internship draws talent from across the country. Many interns gain friends for life through their summer experience in Minneapolis. In addition to MHIF-organized activities, interns plan activities for the weekends. Past interns have enjoyed camping, hiking, canoeing, BBQs and exploring the Twin Cities of Minneapolis and St. Paul.
Frequently Asked Questions
How many applications do you typically receive? 150-250 each year.
How many research interns are accepted? 7-9 new interns plus one lead intern from the previous cohort.
Should I apply if my GPA is below 3.6? A 3.6 GPA or above is highly preferred. The application has a space to explain extenuating circumstances.
Is a social security number (SSN) required to participate in this research internship? Yes, a SSN is required by the US Internal Revenue Service of all employers who report wages (stipend). Further information regarding SSNs can be found on the Social Security fact page .
Can I submit a letter of recommendation with my application? No. The online application with an attached resume are the accepted items.
The academic calendar for my school ends after Memorial Day or starts before the research internship program ends. Can I still apply? Interns are to be available to participate full-time in the internship from May 28 through August 15, 2025 with the first three days of orientation being key to a successful internship. Explain your circumstances in the eligibility section for consideration.
I anticipate attending summer classes. Can I still apply? This research internship is a full-time endeavor. It is not advised to apply.
What are the typical days/hours for this internship? What if I have a conflict? The internship program begins May 28 through August 15, 2025. MHIF is closed on May 27 for Memorial Day, June 19 and July 4. If interns have an important conflict, it should be pre-approved and should be limited to no more than 1-2 business days during the internship.
Core intern work days/hours are Monday to Friday from 7:30 am to 4:00 pm. Projects may require additional hours as deadlines approach. There are also some evening events or early morning observations.
I will be applying to medical school or doing medical school interviews during the summer. Can I still apply? Completing medical school applications is acceptable and needs to be completed outside of the research internship work hours. Attending medical school interviews are possible as long as the intern fulfills the expectations for the internship, which can require additional hours over and above scheduled work hours.
What statistical and software skills are needed? Interns will be using statistical software (including R), Excel, Word, and PowerPoint to build and maintain data dictionaries, do data cleaning, data validation, data verification, and work with demographic data. While some training will be provided, a working knowledge of these tools will be helpful.
Do you provide housing support? Generally we are not able to offer housing support. However, we share intern contact information for roommate collaborations and recommend researching the University of Minnesota campus (approximately two miles away), Facebook and Craigslist for short-term rental or sublet options. Scholarship opportunities may be available for accepted applicants who need housing/transportation support.
Can I apply again, if I am not selected this time? Yes, as long as you still meet the eligibility requirements.
Where can send a question if my question is not answered here? Email [email protected]
2025 Internship Application
Applications for the 2025 cohort will open on November 8th - check back for more details.
Cheers to 20 Years of Interns!
During the past 20+ years, 244 clinical research interns have contributed to 223 posters and presentations at national scientific sessions, 219 publications in peer-reviewed journals, and 59% of former interns are now practicing physicians !
In celebration of this 20-year landmark, we were excited to reconnect with Dr. Aaron Tande, MD , who was part of our first internship program in 2002 . Hear what Aaron has to say about his internship experience 20 years later:
Intern Program Highlights
Since 2002, 244 research interns have contributed to….
223 posters and presentations at national scientific sessions
219 publications in peer-reviewed journals
Where are they now.
59% are physicians
24% are in medical school
12% are in healthcare related fields
5% are in non-healthcare fields

Intern Experience
During the summer research internship, interns will be exposed to a vast array of clinical research and educational experiences. They will be matched with a physician and staff mentor, who will guide them through a clinical research project throughout the summer. This valuable exposure takes place at a nationally ranked and award-winning hospital, Abbott Northwestern Hospital in Minneapolis, Minnesota.
In addition to many observations of surgery and procedures in the hospital, interns will embark on several field trips to destinations including the University of Minnesota Heart Lab, medical device companies and research organizations. Weekly Lunch and Learn presentations from the renowned cardiologists at the Minneapolis Heart Institute® and other guest speakers will offer exposure to the wide range of research happening at MHIF and unique career paths.
Meet Our Interns
Meet our 2024 Interns >
Meet our 2023 Interns >
Meet our 2022 Interns >
Meet our 2021 Interns >
Meet our 2020 Interns >
Meet our 2019 Interns >

2024 Intern Program Donors
This cutting-edge educational experience is made possible by the generous contributions of individual donors, local foundations and industry organizations who share in the belief that hands-on experience is critical for tomorrow’s leaders. Financial support from donors means interns can work with both a staff and a physician mentor to learn about the research process and cardiology.
Thank you to the following generous donors for their contributions to the 2024 MHIF Internship Program:
|
Heart Rhythm Intern Cline and Dianne Hickok Intern Leonardus Loos and Shelley Holzemer Intern Dubes Family Intern Pete Pierce Intern Rose Peterson Memorial Intern Discovery Intern Drs. Richard and Jeanette Hoffman Intern |
M. Nicholas Burke, MD & Sue Slattery-Burke Ivan Chavez, MD & Gail Chavez Leah and Patrick Coveney Jerry and Charlotte Golinvaux Family Fund (Bank of America) Meghan and Russel Holmes Hopkins Youth Hockey Association David Hurrell, MD & Stacey Hurrell Michael and Cara Jennings Minnesota Hospital Association Charles and Shirley Paterson Denise Windenburg and Jill Schwimmer |

Support the Intern Program
Help fund the next generation of cardiovascular experts!

The Minneapolis Heart Institute Foundation® (MHIF) strives to create a world without heart and vascular disease. To achieve this bold vision, we are dedicated to improving the cardiovascular health of individuals and communities through innovative research and education.
Thanks to the generosity of donors like you , we can continue this life-saving work. Please make a gift to support the area of greatest need.
Brad Paisley at Heart 360: Concert for Heart Research

Ready to celebrate with a community that has heart? Join us for our annual Heart 360 Concert for Heart Research on Saturday, November 2 at The Armory with headliner and award-winning country music singer Brad Paisley and special guest Corey Kent ! Purchase GA tickets via Ticketmaster, or Premium VIP tickets today:
- Podiatrists
- Optometrists
- Primary Care Clinics
- Dental Clinics
- Pediatric Clinics
- Mental Health Clinics
- OBGYN Clinics
- Day Surgery Centers
- Drug Rehabs
- Urgent Care
- Dialysis Centers
- Nursing Homes
- Home Health
- Assisted Living
- Ambulance Services
- Pharmacists
- Medical Supplies
- Psychologists
- Clinical Social Workers
- Marriage Counselors
- Behavioral Health Centers
- Behavior Analysts
- Behavior Technicians
- Case Managers / Coordinators
- Chiropractors
- Physical Therapy
- Occupational Therapy
- Speech-Language Pathology
- Massage Therapy
- Acupuncture
- Audiologists
- Medical Students
- Home Health Aides
- Privacy Policy
Clinical Research Institute, Inc in Minneapolis - Location, Contact
- All Providers
- Specialists
- Minneapolis
| CLINICAL RESEARCH INSTITUTE, INC | |
| 825 Nicollet Mall Ste 1135, , Minneapolis Minnesota, 55402-2700 | |
| 612-349-6478 | |
| Cfo | |
| 612-333-2200 |
| 11/28/2011 | |
| 11/28/2011 |
| 825 Nicollet Mall Ste 1135, | |
| Minneapolis | |
| Minnesota | |
| 55402-2700 | |
| 612-333-2200 | |
| 612-349-6478 |
| Other Service Providers | |
| 1744R1102X | |
| Definition to come... |
Post Comments / Review Below
- All Physicians
- All Dentists
Clinical Research Institute
Updated 3 months ago
Photos & videos
See all 2 photos

Location & Hours
Suggest an edit
825 Nicollet Mall
Minneapolis, MN 55402
Downtown Minneapolis
| Closed now |
Upcoming Special Hours
Thu, Nov 28, 2024 | |
|---|---|
Fri, Nov 29, 2024 | |
Wed, Dec 25, 2024 |
Recommended Reviews
Medical School
- MD Students
- Residents & Fellows
- Faculty & Staff
- Duluth Campus Leadership
- Department Heads
- Dean's Distinguished Research Lectureship
- Dean's Tribute to Excellence in Research
- Dean’s Tribute to Excellence in Education
- Twin Cities Campus
- Office of the Regional Dean
- Office of Research Support, Duluth Campus
- CentraCare Regional Campus St. Cloud
- Office of Diversity, Equity & Inclusion
- Office of Faculty Affairs
- Grant Support
- Medical Student Research Opportunities
- The Whiteside Institute for Clinical Research
- Office of Research, Medical School
- Graduate Medical Education Office
- Office of Graduate and Postdoctoral Studies
- Departments
- Centers & Institutes
- Dr. James E. Rubin Medical Memorial Award
- Fisch Art of Medicine Student Awards
- Graduating Medical Student Research Award
- Veneziale-Steer Award
- Dr. Marvin and Hadassah Bacaner Research Awards
- Excellence in Geriatric Scholarship
- Distinguished Mentoring Award
- Distinguished Teaching Awards
- Cecil J. Watson Award
- Exceptional Affiliate Faculty Teaching Award
- Exceptional Primary Care Community Faculty Teaching Award
- Herz Faculty Teaching Development Awards
- The Leonard Tow Humanism in Medicine Awards
- Year 1+2 Educational Innovative Award
- Alumni Philanthropy and Service Award
- Distinguished Alumni Award
- Early Distinguished Career Alumni Award
- Harold S. Diehl Award
- Nomination Requirements
- Duluth Scholarships & Awards
- Account Management
- Fundraising Assistance
- Research & Equipment Grants
- Medical School Scholarships
- Student Research Grants
- Board members
- Alumni Celebration & Reunions
- Alumni Relations - Office of Alumni Relations
- Maximizing Medical Practice Conference
- Diversity & Inclusion
- The Bob Allison Ataxia Research Center
- Children's Health
- Orthopaedics
- Regenerative Medicine
- Scholarships
- Transplantation
- MD Student Virtual Tour
- Why Minnesota?
- Facts & Figures
- Annika Tureson
- Caryn Wolter
- Crystal Chang
- Emily Stock
- Himal Purani
- Jamie Stang
- Jordan Ammons
- Julia Klein
- Julia Meyer
- Kirsten Snook
- Madison Sundlof
- Rashika K Shetty
- Rishi Sharma
- Savannah Maynard
- Prerequisite Courses
- Student National Medical Association Mentor Program
- Minority Association of Pre-Med Students
- Twin Cities Entering Class
- Next Steps: Accepted Students
- Degrees Offered
- Preceptor Resources (MD)
- Doctor of Physical Therapy (DPT)
- Message from the Director
- Clinical Training
- Curriculum & Timeline
- Integrated Physician Scientist Training
- MSTP Student Mentoring and Career Development
- External Fellowships
- Student Directory
- Student Advisory Committee
- Staff Directory
- Steering Committee
- Alumni Directory
- MSTP Student First Author Papers
- Thesis Defense
- MSTP Graduate Residency Match Information
- MSTP Code of Ethics
- Research Experience
- International Applicants
- Prerequisites
- Applicant Evaluation
- The Interview Process
- Application Process
- Financial Support
- Pre-MSTP Summer Research Program
- MSTP Virtual Tour
- Physician Scientist Training Programs
- Students with Disabilities
- Clinical Continuity & Mentoring Program
- Mental Health and Well-Being
- Leadership in Diversity Fellowship
- M1 and M2 Research Meeting
- Monthly Student Meetings
- MSTP Annual Retreat
- Preparation
- Women in Science & Medicine
- Graduate Programs/Institute Activities & Seminars
- CTSI Translational Research Development Program (TRDP)
- Grant Opportunities
- Graduate Programs
- Residencies & Fellowships
- Individualized Pathways
- Continuing Professional Development
- CQI Initiative
- Improvement Summary
- Quality Improvement Communication
- Student Involvement
- Student Voice
- Contact the Medical School Research Office
- Funding Opportunities
- Research Ethics
- Research Support
- Training Grants
- Veteran's Affairs (VA)
- ALPS COVID For Patients
- 3D/Virtual Reality Procedural Training
- Addressing Social Determinants of Health in the Age of COVID-19
- Augmented Reality Remote Procedural Training and Proctoring
- COVID19: Outbreaks and the Media
- Foundations in Health Equity
- Global Medicine in a Changing Educational World
- INMD 7013: COVID-19 Crisis Innovation Lab A course examining the COVID-19 crisis
- Intentional Observational Exercises - Virtual Critical Care Curriculum
- Live Suture Sessions for EMMD7500
- M Simulation to Provide PPE training for UMN students returning to clinical environments
- Medical Student Elective course: COVID-19 Contact Tracing with MDH
- Medical Students in the M Health Fairview System Operations Center
- Telehealth Management in Pandemics
- Telemedicine Acting Internship in Pediatrics
- The Wisdom of Literature in a Time of Plague
- Virtual Simulations for EMMD7500 Students
- COVID-19 Publications
- Blood & Marrow Transplant
- Genome Engineering
- Immunology & Infectious Disease
- Magnetic Resonance Imaging
- News & Events
- Support the Institute
- Lung Health
- Medical Discovery Team on Addiction Faculty
- Impact on Education
- Meet Our Staff
- Donation Criteria
- Health Care Directive Information
- Inquiry Form
- Other Donation Organizations
- Death Certificate Information
- Social Security Administration Notification and Death Benefit Information
- Grief Support
- Deceased Do Not Contact Registration and Preventing Identity Theft
- Service of Gratitude
- For Educators and Researchers
- Clinical Repository
- Patient Care
- Blue Ridge Research Rankings
- Where Discovery Creates Hope
Minnesota Clinical Research Network
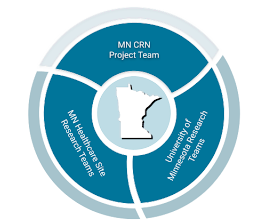
Build on rural Minnesotans’ access to a wide range of human participant research trials by expanding sustained relationships with physicians, clinics, and hospitals in greater Minnesota and ensuring the resources are in place to support that work.
Initiative Information
+ guiding principles.
- Expand the number of mutual relationships with bidirectional input, working to generate equity and trust with host sites – recognizing their expertise and experience.
- Allow healthcare sites and communities to define areas of need and priorities in research — identify UMN researchers and programs that fit with host sites' research interests.
- Select research projects that offer equitable opportunity to be a part of research for all participants regardless of geographic location
- Practice humility, cultural sensitivity, and respect for all involved —respect the host site and local community history, strengths, experience and knowledge
- Be accountable by setting up regular evaluation of programming to reduce unintended consequences or negative outcomes.
- Aim for sustainable research programming by using existing structures that are working – aim to strengthen and build capacity.
+ Goals of the initiative
- Establish sustainable relationships between the UMN and hospitals, clinics, and physicians in Greater MN and with rural MN communities, on an organizational rather than a PI by PI basis.
- Establish supportive collaborations with MN programs and networks engaged in rural healthcare initiatives.
- Support trials that minimize participant travel to the Twin Cities with most of the interactions with research participants occurring in their own home clinic, a nearby clinic or hospital, or virtually.
- Create policies, procedures, and tools to support this work that are tailored to meet the health system’s and community’s needs.
- Create informational documentation about the initiative and ensure that resources are available to UMN faculty to support multi-site trial development, including flexible technology solutions for healthcare sites and travel to greater Minnesota communities to support recruitment, participant visits, and relationship building.
+ What Are We Working on Now?
- Establishing a Scientific Steering Committee to guide the initiative’s research area of focus and relationship building strategy.
- Making connections with other groups, programs, and initiatives in the Greater MN healthcare, community, and research space to learn about their work and identify potential areas of collaboration.
- Developing our strategy for operationalizing the initiative, including the research study intake process, initiative services and cost structure, and our communication plan with researchers, healthcare sites, and other potential collaborators.
- Creating SOPs and other key documentation to support initiative operations and management.
- Planning a pilot study to run through the initiative that longitudinally examines biomarkers of aging in people aged 50-79 living in Greater MN.
+ Selection Criteria for Studies
All studies will be reviewed against the following criteria:
- The researcher has obtained funding to support the study operations/implementation expenses, including the cost of study management at partnering host sites.
- If the researcher is submitting a proposal to the initiative prior to grant submission, the grant application will include a request for funds to support the study operations/implementation expenses, including the cost of study management at partnering host sites.
- The researcher is able to comply with department specific resource review requirements, which may include seeking preliminary resource review as part of a grant partnership within the initiative
- The research under study falls within an area of interest or need by one or more host sites and associated local communities
- The researcher has shown interest or made partnership connections with one or more host site clinicians. Note that this can be through project design and/or grant inception discussions, percolator events, or through conversation with the Research Office.
- The study offers equitable opportunities for eligible participants within the existing clinical population of the host site(s).
- There is staffing capacity to take on the study, or there is an opportunity to hire.
- The proposed study will not negatively impact enrollment for active studies recruiting the same clinical and geographic population.
- There is not another departmental resource or existing infrastructure that may be a better fit to take on the project (e.g., another clinical trial network with expertise in the research area).
- There are no aspects of the study that could pose a general barrier for implementation at greater MN healthcare sites. Note that sites will undergo a detailed feasibility and selection process once the study has been selected for inclusion into the initiative.
+ Operations
- Services and Cost Structure Coming soon
Frequently Asked Questions
+ what type of studies are a good fit for the initiative.
The initiative can support a wide variety of research trials, from observational studies to interventional clinical trials, including drug/device and behavioral interventions. Studies are chosen based on the research interests of clinical and community partners around Greater MN. Some examples of research topics include aging research, mental health, addiction, oncology, diabetes, obesity, Lyme’s disease, although proposals for other research topics are welcome.
+ Which sites in Greater MN has the initiative been in communication with?
The initiative team has had conversations with the following healthcare sites and organizations:
- CentraCare Health System (St. Cloud and Willmar, MN)
- Essentia Institute of Rural Health & St. Mary’s Medical Center (Duluth, MN)
- Fairview Range Medical Center (Hibbing, MN)
- Grand Itasca Clinic & Hospital (Grand Rapids, MN)
- Whiteside Clinical Research Institute & St. Luke’s Hospital (Duluth, MN)
We are also interested in making connections with additional healthcare sites, so if you represent or collaborate with an organization that is not on this list, please feel free to reach out to us to start a conversation.
+ I am a researcher in Greater MN who is not affiliated with UMN. Can I bring my research idea to the initiative?
Yes! This research initiative is designed to create partnerships with researchers across Greater MN. We would be happy to initiate a partnership discussion and meet with you to discuss your research plans and interests.
+ What services do the Greater MN Clinical Research Initiative offer?
The Greater MN Clinical Research Initiative is intended to support initiation and conduct of research at participating greater MN institutions by providing project management oversight throughout the life cycle of a project. This oversight includes activities such as supporting partnership initiation, facilitating research implementation discussions, providing guidance on multisite study management and supporting ongoing communication with partner sites. We will review each project with the investigator to determine the appropriate level of support based on the needs of the research study.
+ Can I work with my departmental research personnel on a study that I bring to the initiative?
Yes. Researchers should utilize their existing departmental research support structures when feasible, and we will collaborate with your team to advance your project. Please contact us if you have any questions about study resourcing.
+ I need support in developing and running a multisite clinical trial protocol. Can the initiative help with this?
Yes! Greater MN Clinical Research Initiative team members have experience in managing multicenter research trials and have developed processes and documentation for efficient communication and oversight of clinical trial sites.
Interested in partnering with us?
- Email: [email protected]
Leadership:
- Dr. Shailey Prasad, Associate Vice President for Global and Rural Health, OACA
- Dr. Diane Treat-Jacobson, Associate Dean for Research, School of Nursing
Medical School Staff Support
- Jane Welter, Senior Director of Research Strategy and Operations, [email protected]
- Stephanie Preekett, Senior Project Manager, [email protected]
Office of Academic Clinical Affairs
Clinical and Translational Science Institute
- CTSA Application 2023
- What is Translational Research?
- Early Career Research Award
- Internal Advisory Board
- Clinics and Surgery Center (CSC)
- Informatics
- Management Council
- Office of Discovery and Translation (ODAT)
- About the Rural Health Program
- Workforce Development (TWD)
- Clinical Research Support Center (CRSC)
- How Billing Works
- Budgets & Finances
- Consultations & Matching
- Forums for Community Input
- Tools & Resources
- Find & Access Data
- Work With Your Data
- Trial Innovation Network (TIN)
- Accrual to Clinical Trials (ACT) Network
- Midwest Area Research Consortium for Health (MARCH)
- Recruitment
- Regulatory Specialists
- Clinical Trial Monitoring
- IND/IDE Applications
- ClinicalTrials.gov Support
- Biospecimen
- Histology & Digital Imaging
- Study Planning & Protocol Support
- More Services (CRSC)
- All Opportunities
- Translational Science
- Translational Research
- Community Health Collaborative Pilot Grant
- Community Partnership Grant
- Child Health
- K Accelerator Program (KAP)
- K12 Scholars Career Development Program
- K-R01 Transition to Independence Program
- K Scholar Multidisciplinary Seminar Series
- K and Beyond Club
- Mothers Leading Science Program
- Northern LITeS: Leadership for Innovative Team Science
- Custom Training Plan
- New Staff Training
- Essentials for Regulatory Specialists
- Informed Consent Workshops
- Online Training
- Seminar Series
- Rural Health Equity Postdoctoral Program
- Translational Research Development Program
- CTSI T32 Program
- Advanced Pathways to Research Program (A-PReP)
- Undergraduates
- Mentor Training
- Become a Mentor
- Mentor of the Year Award
- Community Members
- Community-engaged Teams
- Electronic Data Capture (EDC)
- Feasibility Analysis
- About OnCore
- Studies to Enter into OnCore
- ResearchMatch
- Research Toolkit
- StudyFinder
- Upcoming Events
- Community-engaged research workshops
- Write Winning NIH Grants Annual Seminar
- Past Events
CTSI is enhancing the way research is conducted to make a meaningful impact on people’s lives. We provide a comprehensive infrastructure of research services, training, grants, tools, and more.
Expert guidance for investigators and study teams
- Budgets and finances
- Community engagement
- Data and informatics
- Multi-site studies
- Study planning
More experts and services
Education and training programs
Post-docs and fellows

Funding promising research
We’ve awarded millions in research funds to support hundreds of University of Minnesota faculty investigators and community-University research teams.
Grants from CTSI
Providing research tools and resources
Find tools, guidance, and other resources for conducting health sciences research at the University of Minnesota.
- Electronic data capture
- Feasibility analysis tools
UMN researchers find use of metformin in adults with diabetes linked to lower risk of Long COVID

From crisis to community: A tribe’s response to the opioid epidemic

Research professional training program reaches major milestone
How to develop and sustain a community advisory board for research.
Advertisement
Clinical Research Support Center
- Cite the CRSC

The Clinical Research Support Center is a collaboration among: Clinical and Translational Science Institute; Research and Innovation Office; Fairview Health Services; and University of Minnesota Physicians.
Budget development
Clinical trial monitoring
Community engagement
Data access
Feasibility reviews
IND/IDE determination and support
Institutional Review Board (IRB)
IRB submission preparation
M Health Fairview
OnCore services
Protocol support
Recruitment
REDCap assistance
Sponsored Projects Administration
Statistical support
“This was a fabulous experience. I have been writing UMN IRB protocols for years, and this is the most educational and supportive experience I have had in the past 10 years. Well done, and bravo for the workflow you have generated here.”
— UMN investigator
Saving study start-up time
“[The CRSC] supported our research study in every aspect that we needed them to. When we had questions, they were there to answer them and help streamline this process for us.”
Manda Keller-Ross, PhD, DPT, PT, Division of Physical Therapy
More use cases

CTSI leadership retirement and transition

How to develop UMN research protocols

CRSC celebrates five years

Conduct Your Next Clinical Study with CRI
With nearly 40 years of experience, and over 1,000 phase I, II, III, IV clinical studies conducted in a variety of therapeutic areas, CRI is the right place to conduct your next study.
Decades of Experience Conducting Clinical Studies
CRI conducts studies in a variety of therapeutic areas including vaccines, asthma, allergy, migraine, sinusitis, uticaria, atopic dermatitis, COPD, URI, influenza, pneumonia, and more. Our staff is always dedicated to ensuring the studies are a positive and educational experience for the volunteers.
Experienced and Qualified Research Staff
The CRI facility is fully staffed with experienced staff ready to conduct your next study. Our two Principal Investigators are board-certified physicians, certified in Allergy and Immunology. They have each earned the designation of Certified Physician Investigator (CPI®) as determined by the Association of Clinical Research Professionals (ACRP).
- Principal Investigators
- Sub-Investigators
- Research Manager
- ACRP Certified Research Coordinators
- Research Assistants
- Regulatory Manager
- Quality Assurance Manager
- Marketing Manager
- Recruitment Manager
- Financial Manager
Fully-Equipped Facility in an Urban Location
Our fully staffed and fully equipped research facility is conveniently located in Downtown Minneapolis in the Medical Arts Building.
Facility Assets and Equipment:
- Ambient cold centrifuge for laboratory sample processing
- Separate -20C freezer for storage of investigational product and laboratory samples
- Separate -70C freezer for storage of investigational product and laboratory samples
- ECG equipment on-site
- Secured drug/device storage
- Dedicated workstations to accommodate monitor visits, with wireless access
Clinical Resources
- Capability for extended patient stays up to 12 hours
- Capability to perform IV infusion studies
- Extensive experience with pulmonary function testing including bronchoprovocation studies
- Access to expanded radiology services like CT scans and bone densitometry
- Experience in ambulatory Holter monitoring
- Ability to use central IRB services
Emergency Preparedness
- Close access to Level 1 Trauma Center (0.6 miles) and hospital (2 miles)
- Crash cart, oxygen, emergency medications, defibrillator on-site
- All staff CPR certified
- 24-hour physician on-call
- Self-Identified with FDA per GDUFA requirements
Access to a Large Patient Population
Located in an urban downtown setting, our institute can reach a broad, diverse range of demographics. We have access to over 9,000 patients via our research database alone.
Our principal investigators also have a well-established private medical practice with internists, pediatricians, and nurse practitioners actively treating over 10,000 patients. This provides access to a large population served for allergies, respiratory disorders, migraines, and more.
Our extensive patient networks and urban location allow us to effectively recruit representative study populations across numerous therapeutic areas. Whether your study requires general enrollment or is focused on specific patient groups, we can leverage our resources to meet your goals.
Interested in conducting your next clinical study with CRI?
If you are interested in partnering with CRI for future clinical research studies with CRI, please call 612-333-2200 or fill out the form below.
Interested in volunteering for a study?
As a research volunteer, you play a vital role in advancing groundbreaking science and therapies that improve lives., p: 612-333-2200 f: 612-349-6478, [email protected], clinical research institute, 825 nicollet mall, suite 1135 minneapolis, mn 55402.
All Rights Reserved | Clinical Research Institute | Developed by Vivid Image | Privacy Policy
Find a Trial
Clinical trial facilities in minnesota.
Minnesota is home to several reputable clinical trial facilities like Struthers Parkinson’s Center and Prism Research. These research centers offer a wide variety of clinical trials to meet the needs of Minnesota’s diverse patient population.
Clinical trial facilities in Minneapolis and Rochester offer individuals many chances to participate in research studies. To begin your search, use the form on the right to find nearby clinical research centers in Minnesota such as Mayo Clinic Rochester and Fairview Southdale Hospital .
Use our guide to learn which trials are right for you!
We've found 1,892 clinical trial facilities in minnesota.

Select your condition
Common conditions.
- Atrial Fibrillation
- Breast Cancer
- Constipation
- Dermatology
- Fibromyalgia
- Healthy Studies
- High Blood Pressure (Hypertension)
- High Cholesterol
- Irritable Bowel Syndrome (IBS)
- Migraine Headaches
- Obesity Weight Loss
- Osteoarthritis (OA)
- Renal Impairment / Chronic Kidney Disease
- Skin Cancer
- Smoking Cessation
All Conditions
- Alzheimer Disease
- Athletes Foot
- Benign Prostate Hyperplasia
- Bipolar Disorder
- Bladder Cancer
- Blood Cancer
- Brain Cancer
- Cervical Cancer
- Chronic Obstructive Pulmonary Disease
- Chronic Pain
- Cognitive Studies
- Colorectal Cancer
- Contraception
- Contraception Birth Control
- Contraception Birth Control Patch
- Crohns Disease
- Diabetic Neuropathy
- Digestive Disease
- Diverticulitis
- Eating Disorder
- Endometrial Cancer
- Endometriosis
- Erectile Dysfunction
- Flu (Influenza)
- Food Studies
- Gastroesophageal Reflux Disease
- Gastrointestinal
- Genital Herpes
- Hemorrhoids
- Hepatitis C
- Infectious Disease
- Infertility
- Influenza Vaccine
- Insomnia Sleep Studies
- Iron Deficiency Anemia
- Kidney Cancer
- Liver Cancer
- Low Back Pain/Arthritis Pain
- Lung Cancer
- Major Depression Disorder (MDD)
- Menses Disorders
- Multiple Sclerosis
- Osteoporosis
- Other Indications
- Ovarian Cancer
- Overactive Bladder
- Pancreatic Cancer
- Parkinsons Disease
- Peripheral Vascular Disease
- Polycythemia Vera
- Post-Surgical Pain
- Postherpatic Neuralgia
- Postmenopausal Syndrome
- Premature Ejaculation
- Prostate Cancer
- Psychiatric
- Restless Leg Syndrome
- Rheumatoid Arthritis
- Rheumatology
- Schizophrenia
- Shingles Vaccine
- Skin and Soft Tissue Infections
- Thyroid Cancer
- Tobacco Consumers
- Tobacco Consumers: SNUS, Chew, Dip
- Urinary Tract Infections
- Women's Studies
Transforming discoveries into better patient care
Research areas.
At the Hennepin Healthcare Research Institute (HHRI), we've been investigating the causes of and potential treatments for diseases since 1952. We support and oversee medical research at HCMC, Hennepin Healthcare's acute care and teaching hospital. Our research topics are diverse with a focus on four areas: Acute Care/Trauma, Addiction, Health Services and Infectious Diseases (HIV/AIDS). Innovative discoveries made at HHRI improve the lives of patients in our local community and across the globe.

Acute Care/Trauma

Health Services
Infectious Diseases
Hhri research highlights.

How systemic barriers and social drivers significantly impact health more than other factors
The Health, Homelessness, & Criminal Justice (HHCJ) Lab is nestled within one of the largest medical research nonprofits in Minnesota, Hennepin Healthcare Research Institute (HHRI), which is connected to the Hennepin Healthcare System (HHS). HHCJ’s vision is “Thriving communities without health inequities driven by homelessness or criminal justice involvement.” This is based on robust evidence…

Cause of Death After Prison Release Differs from General Population
from Yale School of Medicine/Internal Medicine Reentry into the community can be an extraordinarily difficult process for the over 1 million adults incarcerated in U.S. prisons. These individuals face substantial barriers and strained social and familial relationships when returning to life outside of jail or prison that contribute to an increased risk of morbidity and…

HHRI in the community and on the air
Hennepin Healthcare Research Institute (HHRI) investigators shared their expertise as guests on radio programs and podcasts in recent months. Here are a few of those interviews. All Things Brains May 26, 2024 Vascular neurologist Behnam Sabayan, MD, PhD, was featured on Hennepin Healthcare’s Healthy Matters – with Dr. David Hilden podcast during Stroke Awareness…
No matter the amount, your donation to medical research makes a big difference in
improving patient care and enhancing lives.
Support advancements in medicine. Donate to HHRI today.

IMAGES
VIDEO
COMMENTS
As a research volunteer, you play a vital role in advancing groundbreaking science and therapies that improve lives. Volunteer Now P: 612-333-2200 F: 612-349-6478
Clinical Research Institute is a dedicated research center located in downtown Minneapolis, MN with a satellite facility located in Plymouth, MN. Founded in 1985, we continue to be a premier research center for the Twin Cities area. Clinical Research Institute is dedicated to promoting patient care through the research of innovative therapies.
A Premier Research Facility Since 1985. CRI is a premier clinical research institute advancing groundbreaking medical therapies through ethical, meticulously conducted studies. For over 35 years, we have been a trusted partner to sponsors and the community in the pursuit of scientific discovery and life-changing treatments.
The Minneapolis Heart Institute Foundation® (MHIF) offers one of the most outstanding and unique internship opportunities available to undergraduates who are pre-med or planning a career in medicine. ... 244 clinical research interns have contributed to 223 posters and presentations at national scientific sessions, 219 publications in peer ...
Dr. Berman has received the Minneapolis/St. Paul Magazine Top Doctor Award in the field of Allergy and Clinical Immunology in 2013, 2014 and 2015. Dr. Berman is active in clinical research acting as principal investigator of studies in asthma, allergy, headache and migraine. He is Past President of Clinical Research Network (CRN) and has served ...
Clinical site with over 5,000 participants. Moral Injury Prevention Program. Survey research and analysis. For more information about working with the Berman Center, please contact Brenda Kirpach at [email protected] or 612-873-6905. You can also visit our website at bermancenter.org. Donate to This Program.
Clinical Research Institute, Minneapolis. 335 likes · 2 talking about this · 9 were here. Seeking volunteers to participate in Clinical Research Studies. We conduct research studies in the Medical...
Clinical Research Institute ( 3 Reviews ) 825 Nicollet Mall, Suite 1135 Minneapolis, Minnesota 55402 (612) 333-2200; Website; Click Here for Special Offer . Listing Incorrect? Listing Incorrect? About; Hours; Details; Reviews; Hours. Thursday: 7:30 AM - 4:30 PM. Friday: 7:30 AM - 4:30 PM.
The NPI Number for Clinical Research Institute, Inc is 1316214885. The current location address for Clinical Research Institute, Inc is 825 Nicollet Mall Ste 1135, , Minneapolis, Minnesota and the contact number is 612-333-2200 and fax number is 612-349-6478.
Specialties: Since 1985, Clinical Research Institute has conducted over 1,000 clinical trials. Our mission is to deliver groundbreaking clinical trials to a diverse population and serve as a healthcare partner to sponsors and our local communities.
Whiteside Clinical Research Institute & St. Luke's Hospital (Duluth, MN) ... The Greater MN Clinical Research Initiative is intended to support initiation and conduct of research at participating greater MN institutions by providing project management oversight throughout the life cycle of a project. ... Minneapolis, MN 55455. BE KIND. PURSUE ...
Clinical and Translational Science Institute. Menu. About. About CTSI. About Us; Impact; CTSA Application 2023; ... the Clinical Research Support Center (CRSC) can hasten the development of therapeutics—not just for one candidate, but for COVID-19 treatments being developed across UMN. ... Minneapolis, MN 55414. Sign Up for Our Newsletter ...
825 Nicollet Mall Ste 1135. Minneapolis, MN 55402. +1 (612) 333-2200. https://criminnesota.com. Since 1985, Clinical Research Institute has conducted over 1,000 clinical trials. Our mission is to deliver groundbreaking clinical trials to a diverse population and serve as a healthcare partner to sponsors and our local communities.
Get started by contacting us at [email protected] or 612-625-4000. Office hours.
If you are interested in partnering with CRI for future clinical research studies with CRI, please call 612-333-2200 or fill out the form below. Interested in volunteering for a study? As a research volunteer, you play a vital role in advancing groundbreaking science and therapies that improve lives.
Minneapolis, MN. Colon And Rectal Surgery Associates. 606 24th Avenue South. Minneapolis, Minnesota 55454. 651-225-7824. mi. from. Minneapolis, MN. Critical Care Research Center at Louisiana State University Health Sciences Center.
About CLINICAL RESEARCH INSTITUTE, INC. Clinical Research Institute, Inc is a provider established in Minneapolis, Minnesota operating as a Specialist with a focus in research study . The healthcare provider is registered in the NPI registry with number 1316214885 assigned on November 2011. The practitioner's primary taxonomy code is 1744R1102X.
Transforming discoveries into better patient care At the Hennepin Healthcare Research Institute (HHRI), we've been investigating the causes of and potential treatments for diseases since 1952. We support and oversee medical research at HCMC, Hennepin Healthcare's acute care and teaching hospital. Our research topics are diverse with a focus on four areas: Acute Care/Trauma, Addiction,…