

What is the ozone layer, and why is it important?
Over the last 50 years, holes in the ozone layer have opened up. why does that matter for life on earth.
One of the most pressing environmental problems over the last century has been the depletion of the ozone layer. But what is the ozone layer, and why does it matter?
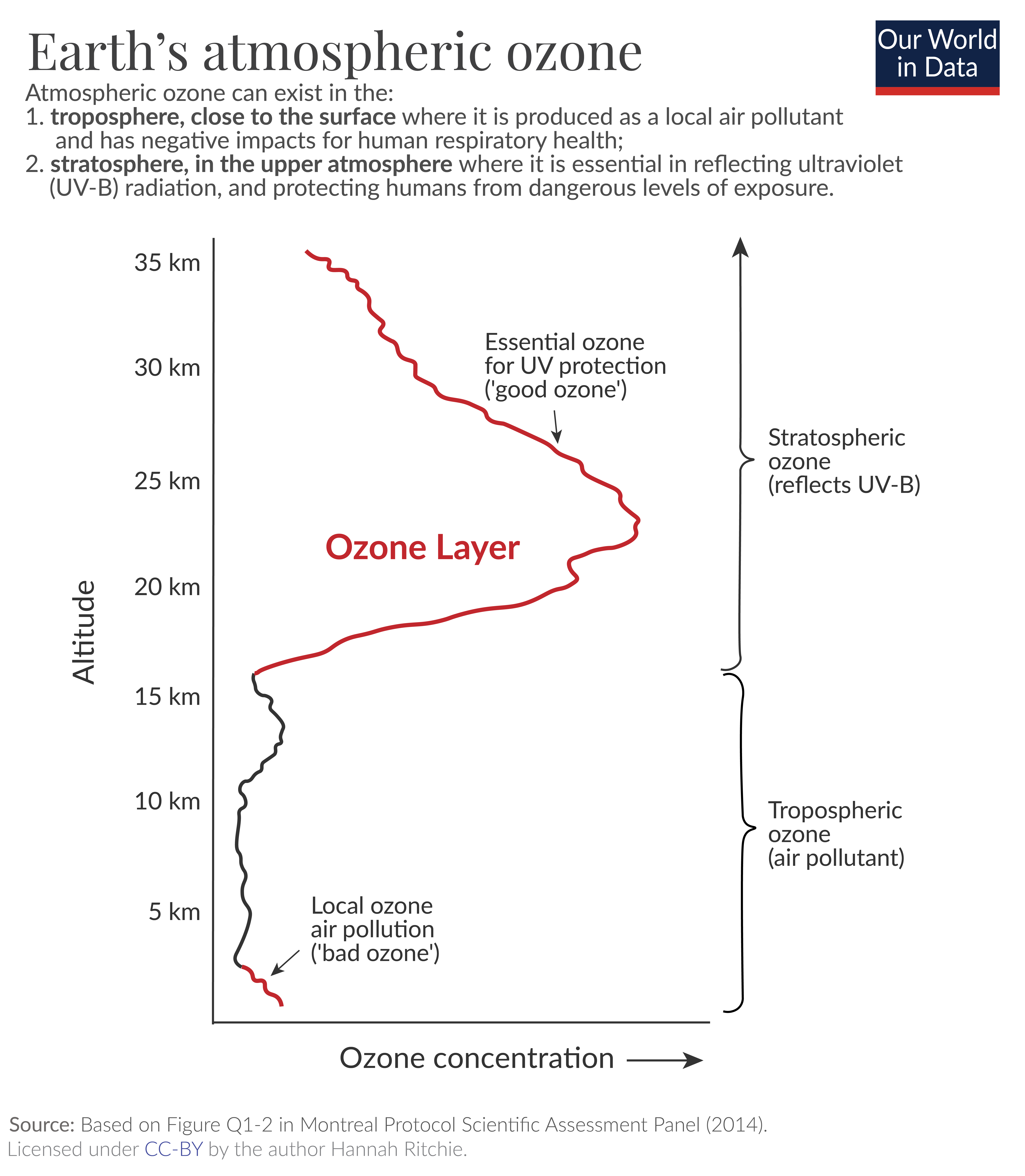
Ozone is a gas present naturally within Earth’s atmosphere. It is formed of three oxygen atoms (giving it the chemical formula O 3 ). Its structure makes it unstable: it can be easily formed and broken down through interaction with other compounds.
Ozone is most highly concentrated at two very different altitudes in the atmosphere: near the surface, and high in the atmosphere (in the stratosphere). Its function is very different in these two zones.
‘Good ozone’ and ‘bad ozone’
Ozone close to the surface is called tropospheric ozone, and it is often referred to as ‘ bad ozone ’. Ozone concentrations are lower in the troposphere than in the stratosphere.
We can see this in the diagram.
However, ozone concentrations close to the Earth’s surface can be temporarily and locally higher, because of emissions from motor vehicle exhausts, industrial processes, electric utilities, and chemical solvents. Ground-level ozone is a local air pollutant, and can negatively impact human health. Breathing ozone is particularly harmful to the young, elderly, and people with underlying respiratory problems.
This is very different from ozone high in the atmosphere: stratospheric ozone. It’s referred to as ‘ good ozone ’.
As shown in the diagram, ozone concentrations are higher in the stratosphere than in the troposphere.
The stratosphere includes the zone commonly called the ‘ozone layer’. It plays a crucial role in keeping the planet habitable by absorbing potentially dangerous ultraviolet (UV-B) radiation from the sun. Before its depletion, the ozone layer typically absorbed 97 to 99% of incoming UV-B radiation.
This means we need high ozone concentrations in the stratosphere to ensure that life — including human life — is not exposed to harmful concentrations of UV-B radiation.
In our work on the ozone layer , we focus on this ozone high in the atmosphere (the ‘good ozone’). The impact of ozone near the surface (‘bad ozone’) is covered in our work on air pollution .
Why is the ozone layer important?
The ozone layer absorbs 97% to 99% of the sun’s incoming ultraviolet radiation (UV-B).
This is fundamental to protecting life on Earth’s surface from exposure to harmful levels of this radiation, which can damage and disrupt DNA.
In the 1970s and ‘80s, humans emitted large amounts of gases that depleted this ozone in the upper atmosphere. As ozone concentrations in the stratosphere fell, and a hole in the ozone layer opened up, there have been measurable increases in the amount of UV-B radiation reaching the surface.
The chart shows the measured change in annual quantities of UV irradiance reaching Earth’s surface, in 2008 compared to 1979. 1
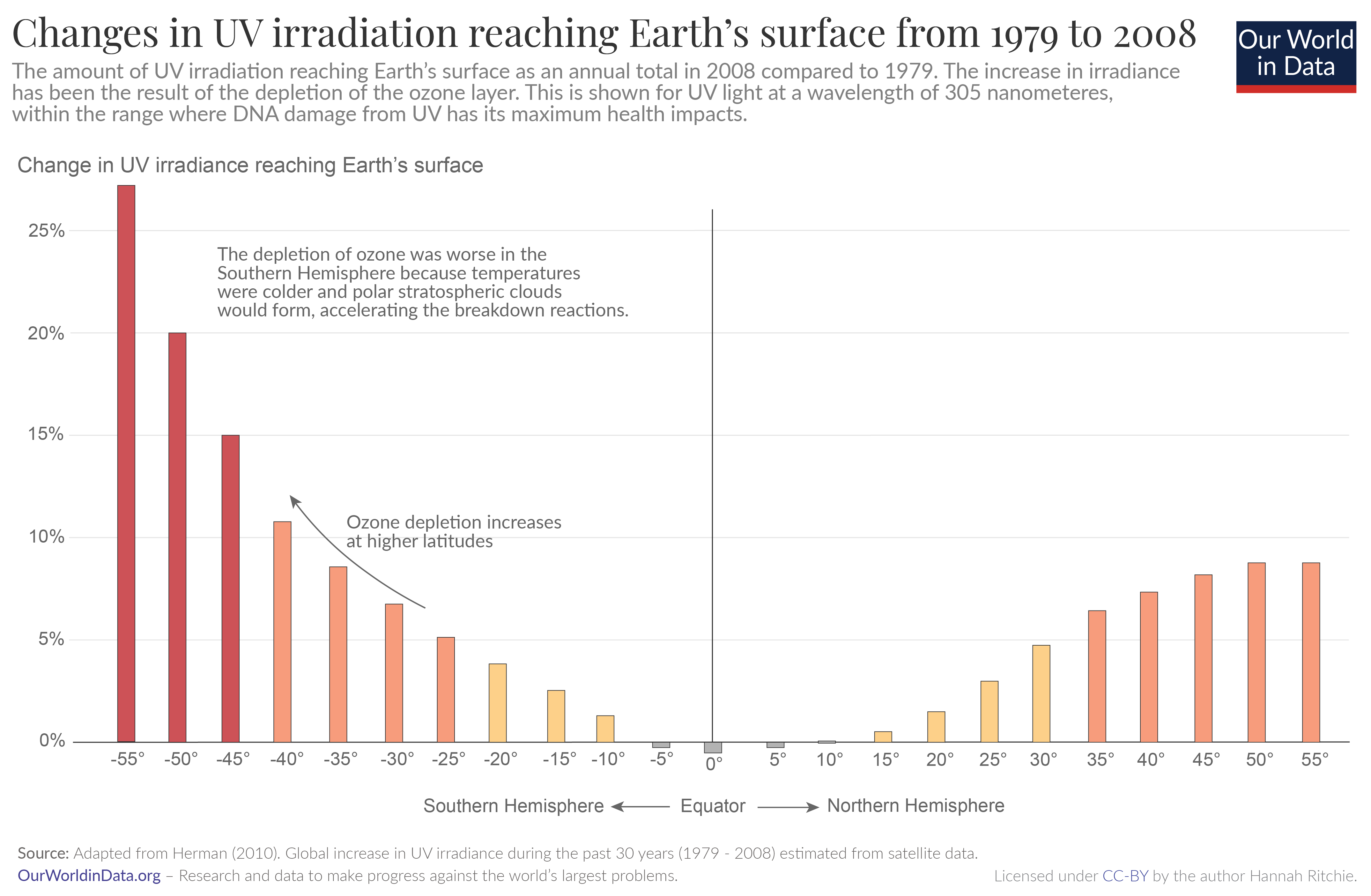
What’s noticeable is that ozone depletion and UV irradiance have increased much more in the Southern Hemisphere. This is because ozone depletion is also impacted by temperature and sunlight. Temperatures are colder at high latitudes in the Southern Hemisphere, so polar stratospheric clouds can form. These clouds can accelerate the reactions that break ozone down.
You will also notice that ozone depletion is worse at higher latitudes. It’s non-existent at the equator, and rises steeply towards the poles. Again, this is influenced by temperature and sunlight. That’s why ozone holes form at the poles, rather than the equator.
This increase in UV-B irradiation reaching the surface matters for life on Earth. One of the biggest concerns has been an increased risk of skin cancer (as well as skin damage and aging). 2 This is because UV-B irradiation can damage skin DNA.
Since the 1980s, the world has achieved rapid progress : the near-elimination of ozone-depleting substances and the trend toward recovering the ozone layer are among the most successful international environmental achievements to date.
Several studies have estimated that millions of excess skin cancer cases have been avoided due to the Montreal Protocol and its follow-up treaties. 3
This is given for UV at a wavelength of 305 nanometers (nm), which is well within the range where it has maximum damage to DNA.
Herman, J. R. (2010). Global increase in UV irradiance during the past 30 years (1979–2008) estimated from satellite data . Journal of Geophysical Research: Atmospheres, 115(D4).
Pitcher, H. M., & Longstreth, J. D. (1991). Melanoma mortality and exposure to ultraviolet radiation: an empirical relationship . Environment International, 17(1), 7-21.
Clydesdale, G. J., Dandie, G. W., & Muller, H. K. (2001). Ultraviolet light induced injury: immunological and inflammatory effects . Immunology and Cell Biology, 79(6), 547.
Dijk, A., Slaper, H., den Outer, P. N., Morgenstern, O., Braesicke, P., Pyle, J. A., & Tourpali, K. (2013). Skin Cancer Risks Avoided by the Montreal Protocol—Worldwide Modeling Integrating Coupled Climate: Chemistry Models with a Risk Model for UV . Photochemistry and Photobiology, 89(1), 234-246.
Slaper, H., G. J. M. Velders, J. S. Daniel, F. R. de Gruijl and J. C. van der Leun (1996) Estimates of ozone depletion and skin cancer incidence to examine the Vienna convention achievements . Nature 384(6606), 256–258.
Cite this work
Our articles and data visualizations rely on work from many different people and organizations. When citing this article, please also cite the underlying data sources. This article can be cited as:
BibTeX citation
Reuse this work freely
All visualizations, data, and code produced by Our World in Data are completely open access under the Creative Commons BY license . You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
The data produced by third parties and made available by Our World in Data is subject to the license terms from the original third-party authors. We will always indicate the original source of the data in our documentation, so you should always check the license of any such third-party data before use and redistribution.
All of our charts can be embedded in any site.
Our World in Data is free and accessible for everyone.
Help us do this work by making a donation.
FREE SHIPPING ON & ABOVE ₹ 999
- NCERT Solutions Class 9
- NCERT Solutions Class 10
- NCERT Solutions Class 11
- NCERT Solutions Class 12
- NCERT Books Class 6
- NCERT Books Class 7
- NCERT Books Class 8
- NCERT Books Class 9
- NCERT Books Class 10
- NCERT Books Class 11
- NCERT Books Class 12
- NCERT Exemplar Class 9
- NCERT Exemplar Class 10
- NCERT Exemplar Class 11
- NCERT Exemplar Class 12
- CBSE Notes Class 9
- CBSE Notes Class 10
- CBSE Notes Class 11
- CBSE Notes Class 12
- CBSE Class 9
- CBSE Class 10
- CBSE Class 11
- CBSE Class 12
- Sample Paper Class 10
- Sample Paper Class 12
- CBSE Question Bank Class 9
- CBSE Question Bank Class 10
- CBSE Question Bank Class 11
- CBSE Question Bank Class 12
- Previous Year Question Papers Class 10
- Previous Year Question Papers Class 12
- CBSE Practice Papers Class 10
- CBSE Practice Papers Class 12
- CBSE Worksheets Class 6
- CBSE Worksheets Class 7
- CBSE Worksheets Class 8
- CBSE Worksheets Class 9
- CBSE Worksheets Class 10
- ICSE Class 9
- ICSE Class 10
- Sample Paper Class 9
- ISC Class 11
- ISC Class 12
- Maharashtra SSC Exam Pattern
- Maharashtra HSC Exam Pattern
- Maharashtra SSC Syllabus
- Maharashtra HSC Syllabus
- Maharashtra SSC Previous Year Question Papers
- Maharashtra HSC Previous Year Question Papers
- Maharashtra SSC Question Bank
- Maharashtra HSC Question Bank
- UP Board Class 10
- English Grammar
- Sample Papers
- Solved Papers
- Question Bank
- Model Specimen Papers
- Complete Course
- Competitive
- Key2Practice
Hurry Up! Diwali Offer Live Now – Celebrate with Great Savings! Get Now
Hurry Up! 🪔 Diwali Offer Live Now, Celebrate with Great Savings! Get Now
Essay on Ozone Layer Depletion in English for Students
- September 26, 2023
- Read Time: 9 Minutes
- Publish Date: September 7, 2023
Among the World Ozone Day essay writing topics, ozone layer depletion and its measures is an essential one. Drafting an ozone layer depletion essay requires us to first understand what is an ozone layer.
The ozone layer is a region of Earth’s stratosphere containing relatively high ozone concentration (O3) molecules. The ozone layer plays a significant role in protecting life on Earth by absorbing and blocking a significant portion of the sun’s harmful ultraviolet (UV) rays. This absorption of UV radiation helps protect living organisms from the harmful effects of excessive UV exposure, like skin cancer, cataracts and damage to ecosystems.
Human activities, particularly the release of certain chemicals called ozone-depleting substances, have led to the thinning of the ozone layer in some regions, resulting in the ozone hole.
In this ozone layer depletion essay, we will discuss the various factors contributing to the depletion and amendments that can be adopted to control the same.
- ▪ What is an Ozone Layer?
- ▪ Causes of Ozone Layer Depletion
- ▪ Consequences of Ozone Layer Depletion
- ▪ Global Initiatives to Fight Ozone Layer Depletion
- ▪ Future Predictions and Steps Forward
- ▪ Role of Man in Protecting Ozone Layer
- ▪ Debunking the Myths on Ozone Layer Depletion
- ▪ Wrapping Up
- ▪ FAQs on Ozone Layer Depletion Essay

What is an Ozone Layer?
One of the major components in an ozone layer depletion essay is the composition of this layer and understanding where it is located. Let’s move ahead!
The ozone layer is primarily composed of ozone molecules. It is situated in the Earth’s stratosphere. This layer forms naturally in the stratosphere when oxygen molecules (O2) are exposed to high-energy ultraviolet (UV-C) radiation, causing them to split into individual oxygen atoms. These oxygen atoms can then react with other oxygen molecules to form ozone (O3). This continuous ozone creation and destruction maintains the ozone layer’s balance.
The ozone layer protects the Earth by absorbing and blocking a significant portion of the sun’s harmful ultraviolet radiation. Here’s how it works:
1. Absorption of UV-B and UV-C radiation: The ozone molecules in the ozone layer are particularly effective at absorbing UV-B and UV-C radiation, which are high-energy forms of ultraviolet light emitted by the sun. When these high-energy UV rays strike ozone molecules, they are absorbed and their energy is converted into heat.
2. Preventing harmful UV radiation from reaching the Earth’s surface : By absorbing and dispersing UV-B and UV-C radiation, the ozone layer acts as a shield, preventing a significant portion of these harmful rays from reaching the Earth’s surface. This is crucial because excessive exposure to UV radiation can have harmful effects on living organisms, including humans.
3. Protection against health risks: The ozone layer’s protective role is vital for safeguarding human health. UV-B radiation, for example, is known to cause various health issues such as sunburn, skin cancer, cataracts, and immune system suppression. If not absorbed by the ozone layer, UV-C radiation could be extremely harmful, but fortunately, it is almost entirely absorbed by the ozone molecules.
Long thing short, the ozone layer acts as a natural sunscreen for the Earth, absorbing and blocking harmful UV radiation from the sun, which helps prevent a range of health and environmental risks linked with prolonged UV exposure.
Causes of Ozone Layer Depletion
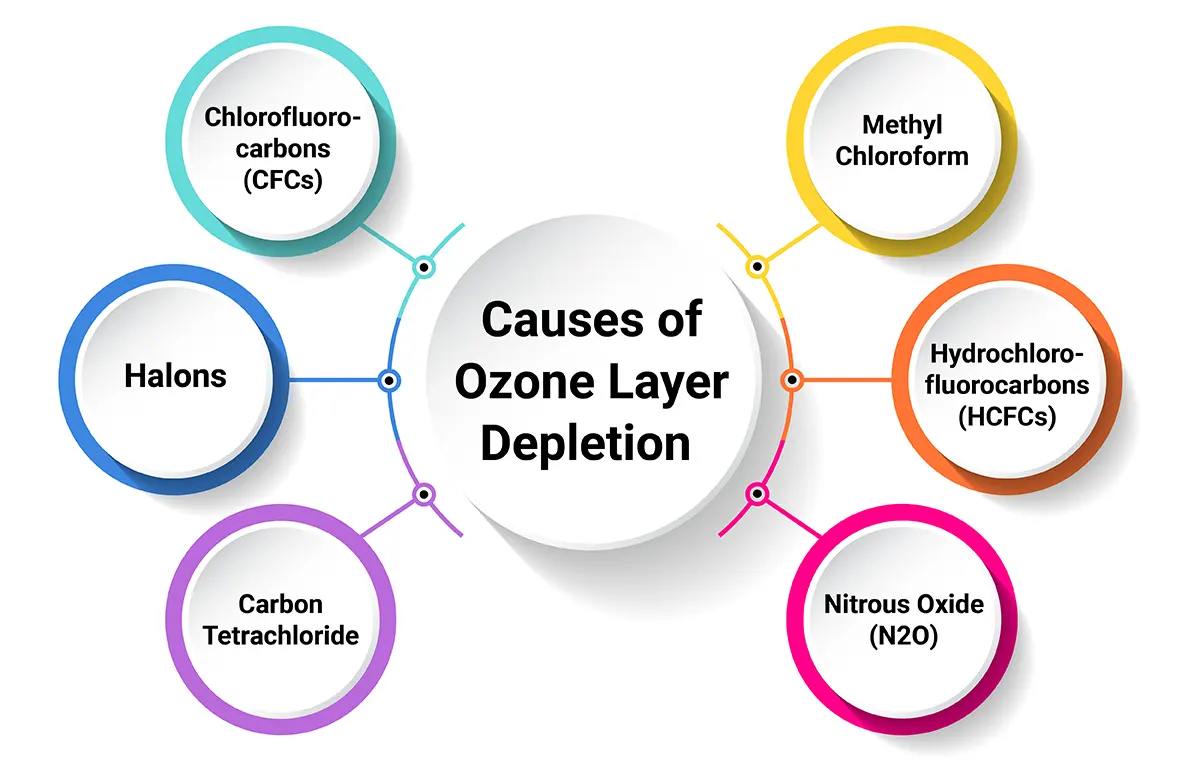
In this ozone layer depletion essay, it’s important to note the various causes that are resulting in ozone depletion. Causes are both natural and man-made, and now, let’s have a look at them.
Manmade causes of ozone layer depletion primarily involve the release of certain chemicals into the atmosphere, which can break down ozone molecules. The most significant contributors include:
1. Chlorofluorocarbons (CFCs) are a major reason for depletion: CFCs were commonly used in refrigerants, aerosol propellants and foam-blowing agents. When released into the atmosphere, they break down in the stratosphere, releasing chlorine atoms that destroy ozone molecules.
2. Halons : Among the major causes of Ozone layer depletion is the use of Halons. These were used in fire extinguishers. Like CFCs, they release bromine and chlorine when they break down, which are ozone-depleting substances.
3. Carbon Tetrachloride: This chemical is used in the production of CFCs and as a solvent. It releases chlorine when it breaks down.
4. Methyl Chloroform: It is used as a solvent, and in industrial processes, it releases chlorine, contributing to ozone layer depletion.
5. Hydrochlorofluorocarbons (HCFCs): While less harmful than CFCs, HCFCs are still ozone-depleting substances used in air conditioning and refrigeration systems.
6. Nitrous Oxide (N2O): This gas, emitted from agricultural and industrial activities, can also contribute to ozone depletion in the stratosphere.
Now, let’s look at a few natural ozone depletion causes that you can add in the ozone layer depletion essay.
1. Solar Variability: Changes in solar radiation and sunspot activity can influence the amount of ultraviolet radiation reaching the Earth’s atmosphere. However, these variations are relatively small and have a limited effect on ozone depletion.
2. Volcanic Eruptions : Large volcanic eruptions can strike sulphur compounds into the stratosphere. These sulfuric acid aerosols can eventually lead to localised ozone depletion, but the effect is generally short-lived and limited in scope.
3. Cosmic Rays: High-energy cosmic rays from space can interact with atmospheric molecules, thus producing reactive nitrogen oxides that can influence ozone chemistry. The contribution of cosmic rays to ozone layer depletion is considered to be minimal.
4. Stratospheric Changes: Natural processes within the stratosphere, such as the dynamics of stratospheric circulation patterns and temperature fluctuations, can influence ozone levels to an extent. However, these processes are interconnected with human-produced ozone depletion and are not independent of natural causes.
Consequences of Ozone Layer Depletion
The next important segment in the Ozone layer depletion essay is the possible consequences of layer depletion. Have a look!
Ozone layer depletion has significant consequences for both the environment and human health. Some of the key consequences include:
1. Increased Ultraviolet (UV) Radiation: Depletion of the ozone layer allows more UV radiation to reach the Earth’s surface. This increased UV radiation can have several detrimental effects:
- Skin Cancer: Higher UV levels are linked to an increased risk of skin cancer, including melanoma.
- Cataracts: UV radiation exposure can contribute to the development of eye cataracts.
- Weakened Immune System: UV radiation can suppress the immune system, making individuals more susceptible to diseases.
2. Damage to Marine Ecosystems: UV radiation can penetrate the ocean’s surface and harm marine ecosystems. It can affect phytoplankton, which form the base of the marine food web, and disrupt aquatic ecosystems.
3. Harm to Terrestrial Ecosystems: Increased UV radiation can damage terrestrial plants, reducing crop yields and affecting natural vegetation. This can impact food production and ecosystem stability.
4. Disruption of Aquatic Food Chains: UV radiation can harm aquatic organisms, including fish larvae, zooplankton, and amphibians, disrupting aquatic food chains and ecosystems.
5. Ozone Holes: In some places, especially over Antarctica, severe ozone depletion forms “ozone holes.” These holes can persist for extended periods, allowing intense UV radiation to reach the surface and causing acute environmental and health risks.
6. Climate Change Issues: Ozone depletion can impact atmospheric circulation patterns and climate. It can also indirectly contribute to global warming because some ozone-depleting substances, like CFCs, are also potent greenhouse gases.
7. Agricultural Impacts degradation: Increased UV radiation can harm crops and reduce agricultural yields, leading to potential food shortages and economic losses.
Global Initiatives to Fight Ozone Layer Depletion
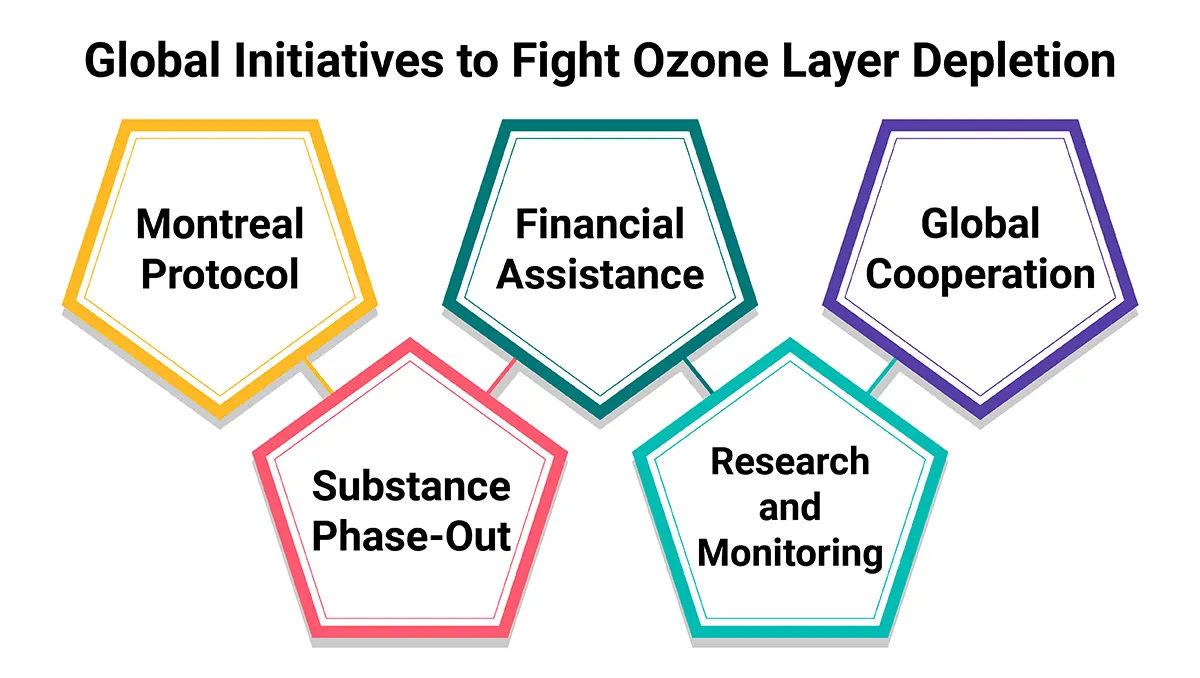
The depletion of the ozone layer is caused by various manmade and natural factors, and here’s how global initiatives are taken to battle the problem.
Global initiatives to combat ozone layer depletion in your ozone layer depletion essay. The most significant initiative is the Montreal Protocol on Substances that Deplete the Ozone Layer, which was adopted in 1987 and has been amended several times to strengthen its provisions. Here are some key elements of these global initiatives:
1. Montreal Protocol: The Montreal Protocol is an international treaty aimed at phasing out ozone-depleting substances (ODS) production and consumption. It sets specific schedules and targets for the phase-out of these substances. Almost all countries in the world have ratified the Protocol.
2. Substance Phase-Out: The Protocol initially focused on the phase-out of chlorofluorocarbons (CFCs), halons, carbon tetrachloride, and other major ODS. Subsequent amendments added additional substances like hydrochlorofluorocarbons (HCFCs) and methyl chloroform.
3. Financial Assistance: To help developing countries transition from ODS, the Protocol established the Multilateral Fund to implement the Montreal Protocol. This fund provides financial and technical assistance to developing countries to support their efforts in phasing out ODS.
4. Research and Monitoring: The Protocol encourages research and monitoring activities related to ozone depletion and the identification of ozone-friendly alternative substances and technologies.
5. Global Cooperation: The success of these initiatives is a testament to global cooperation in addressing environmental challenges. Countries, industries, and environmental organisations have worked together to implement the Protocol’s provisions.
These global initiatives to fight ozone layer depletion have been widely regarded as a success story in international environmental cooperation.
Future Predictions and Steps Forward
In the next segment of the ozone layer depletion essay, write about controlling the ozone layer depletion and the key steps it involves the key steps. It’s a global concern now, as we are solely responsible for protecting Mother Earth and her dear people from the harsh consequences of the depletion.
With new technological innovations and research coming up, new methods can be implemented to resist the depletion. However, humans play an integral role in controlling harmful chemicals directly linked with affecting the ozone layer.
Role of Man in Protecting Ozone Layer
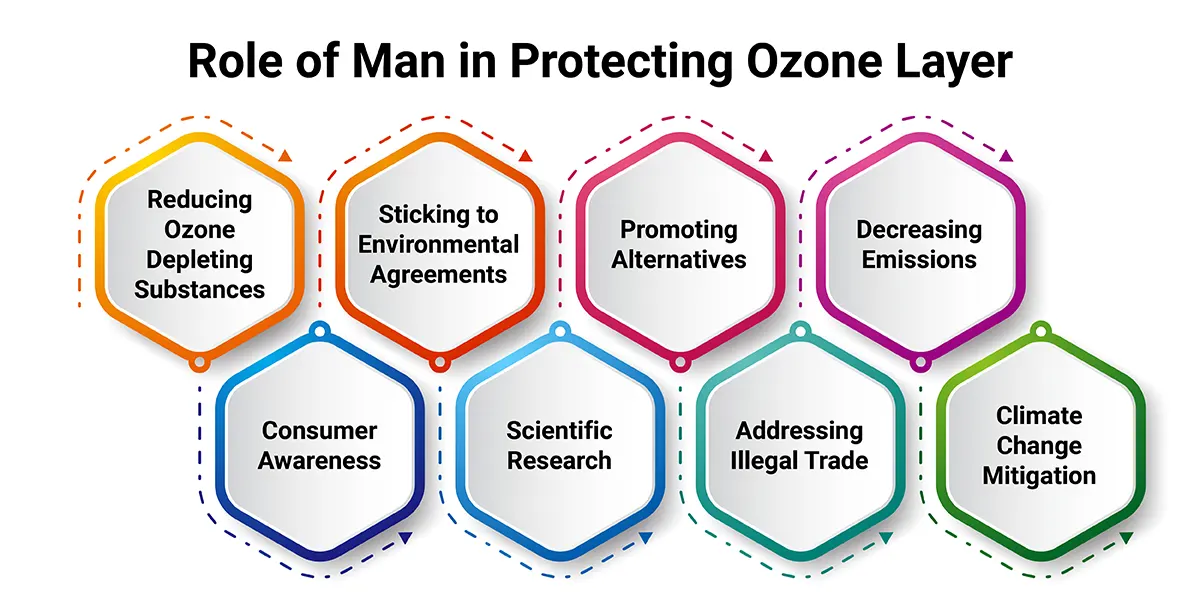
We have discussed several issues in this ozone layer depletion essay, and now let’s look at how humans can contribute towards protecting the ozone layer and safeguarding our mother Earth.
Human actions play a crucial role in protecting the ozone layer. The primary ways in which we can contribute to ozone layer protection include:
1. Reducing Ozone-Depleting Substances: The most significant human contribution is reducing ozone-depleting substances, such as chlorofluorocarbons (CFCs), halons, and carbon tetrachloride. This is achieved through international agreements like the Montreal Protocol, which phases out the production and consumption of these substances.
2. Sticking to Environmental Agreements: Governments and industries should comply with international agreements and regulations, like the Montreal Protocol, to control the production and use of ozone-depleting chemicals. Enforcement of these agreements is essential to limit the release of harmful substances into the atmosphere.
3. Promoting Alternatives: Encouraging the development and use of ozone-friendly alternatives to ozone-depleting substances in various applications, such as air conditioning, refrigeration and aerosol propellants.
4. Decreasing Emissions: Preventing leaks and emissions of ozone-depleting chemicals during manufacturing, transportation, and disposal of equipment containing these substances.
5. Consumer Awareness: Educating the public about the importance of protecting the ozone layer and encouraging responsible consumption and disposal of products containing ozone-depleting substances.
6. Scientific Research: Supporting scientific research to understand better the ozone layer, its depletion, and recovery processes, which can inform policy-making and mitigation efforts.
7. Addressing Illegal Trade: Combating the illegal trade of ozone-depleting substances can undermine international efforts to protect the ozone layer.
8. Climate Change Mitigation: Recognising the link between ozone layer protection and climate change mitigation, as some ozone-depleting substances are also potent greenhouse gases. Addressing both issues together can yield significant environmental benefits.
Human actions, both at the individual and societal levels, are critical in preserving and restoring the ozone layer. International cooperation and continued vigilance are a must to ensure its long-term protection.
Debunking the Myths on Ozone Layer Depletion
There’s no denying that tons of myths revolving around Ozone Layer depletion need to be stopped. Some of the common myths are:
- The Antarctic Ozone “hole” was present all along; it was discovered in the 1970s because that’s when satellite measurements began.
- Volcanoes and other natural causes contribute much more chlorine to the ozone layer than CFC.
- CFCs are unable to reach the stratosphere because they’re heavier than air.
All these misconceptions revolve around Ozone, but recent study findings have helped people gather relevant data about it.
Wrapping Up
With that, we conclude the ozone layer depletion essay. While progress has been made to control the depletion, complete restoration of the ozone layer will take several more decades. Continued adherence to international agreements like the Montreal Protocol and ongoing research and monitoring is crucial to prevent further depletion and ultimately let the ozone layer heal.
So, while we can’t stop it overnight, we can stick to the environmental guidelines for a better tomorrow. Global solid efforts have effectively mitigated ozone layer depletion and offer hope for its eventual recovery.
Also Read: World Ozone Day
FAQs on Ozone Layer Depletion Essay
Q1. what measures can you take to reduce ozone layer depletion at home.
Most chemical-based clinical products include chlorine and bromine that finds a way to reach the atmosphere. Switching to more natural cleaning products can save the ozone layer.
Q2. What can the government do to minimise ozone layer depletion?
The government can ban the use of nitrous oxide or take action against its usage to minimise ozone layer depletion. Mass awareness should be spread too to minimise the usage.
Q3. What are the greenhouse gases that cause ozone layer depletion?
Carbon dioxide, CFCs, methane, nitrous oxide.
Q4. How many oxygen atoms make up the ozone gas?
Three oxygen atoms.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Books Categories
- Login / Register
Username or email address *
Password *
Lost your password? Remember me
Or login with
No account yet?
- Environmental Chemistry
- Ozone Layer
Ozone Layer Cycle
What is the ozone layer.
Almost always the ozone has only been correlated with the hole in the ozone layer and the damages it has caused to the environment. The richness of the ozone layer that makes the hole so significant and the science behind the hole is far less popular. Schönbein in the year 1840 confirmed its existence and Jacques-Louis Soret rooted the chemical formula of ozone as O 3 and proved that ozone is an allotropic form of Oxygen .
Table of Content
- Preparation of ozone
Ozone Structure
Properties of ozone, importance of the ozone layer, ozone layer depletion, recommended videos.
- Frequently Asked Questions – FAQs
The importance of ozone is defined by the fact that it protects the earth from harmful ultraviolet rays from the sun. The ozone layer is found in the upper regions of the stratosphere where it protects the earth from the harmful ultraviolet rays of the sun. These radiations can cause skin cancer in humans. The ultraviolet rays split the oxygen molecule into free oxygen atoms, these free oxygen atoms combine with the oxygen molecule to form ozone. This salient layer lies at a distance of 12-15 miles beyond the earth surface.

Preparation of Ozone
This allotropic form of oxygen is formed by passing dry oxygen through a salient electric with the oxygen molecule to give 5-10% of the allotropic form of oxygen. The product obtained is called ozonized oxygen.
O 2 +O → O 3
3O 2 ⇌ 2O3 – energy (endothermic reaction)
Ozone is unstable and decomposes to molecular oxygen. A dynamic equilibrium is maintained between the formation and decomposition of ozone. It has been found that this protective ozone layer is getting depleted because of the presence of CFC (chlorofluorocarbon) compounds.
When CFC is released into the atmosphere, they mix with atmospheric gases and reach the stratosphere. In the presence of ultraviolet rays, they are broken down to form chlorine radicals. This chlorine radical reacts with ozone to form chlorine monoxide and an oxygen molecule.
CF 2 Cl 2 (g) → Cl (g) + CF 2 Cl (g)
(Note: Cl is in the form of radical)
Cl (g) + O 3 (g) → ClO (g) + O 2 (g)
This reaction breaks down the ozone. CFC compounds are agents which release chlorine radicals into the atmosphere and cause damage to the ozone layer.

Ozone is a polar molecule and to understand this we need to have a look at the structure of Ozone. Ozone resonates between two structures which are shown below:

The middle Oxygen atom has a formal charge of +1 and the atoms at the edge have a formal charge of -1. Due to the separation of light charges and its bent geometry, it has polarity and is considered a polar molecular.
- Ozone in its pure state is blue which has a strong disturbing smell but in a limited proposition, it has a pleasant smell.
- It has the ability to absorb the UV rays which occupy the ultraviolet region which ranges between 220-290 nm of the atmospheric spectrum.
- This form of oxygen boils at 161.2K and forms violet-blue crystals when solidified. It melts at 80.6k.
- This allotrope is a strong oxidizing agent as ozone is an unstable compound under normal conditions and it decomposes quickly in the presence of heat to form nascent oxygen and molecule of oxygen.
Ozone is harmful at ground level but high up the atmosphere ozone layer plays a vital role in the protection of all living beings. The sun propagates ultraviolet radiations which as an adverse effect on living beings. This layer absorbs the radiations and prohibits them from entering the outer surface of the earth. The ozone layer resides in the stratospheric layer of the earth’s atmosphere. The layers which occupy the lower part of the atmosphere removes the unwanted pollutants from the earth’s surface.

The reason behind the ozone layer depletion is mainly due to the extensive use of ozone-depleting substances (ODS). Some ozone-depleting substances are:
- Chlorofluorocarbons (CFC) : The use of CFC’s is one of the main reasons for the depletion of the layer. They are usually used as a coolant in refrigerators and air conditioners used in cars etc. It is also used as an industrial solvent, foam products and as hospital sterilization equipment.
- Methyl chloroform : Finds its applications usually in industries for chemical processing etc.
- Carbon tetrachloride : Normally used as a solvent.
What Is Ozone Layer Depletion And Its Effects?

Frequently Asked Questions – FAQs
What happens when ultraviolet light strikes the ozone layer.
In the stratosphere, ozone is mainly emitted by ultraviolet radiation. When even low-energy ultraviolet radiation is absorbed by an ozone molecule, it breaks into an ordinary oxygen molecule and a free atom of oxygen. Typically, this free oxygen atom easily re-joins to form another ozone molecule with an oxygen molecule.
What are the 2 types of ozone?
Ozone molecules (O3) have three atoms of oxygen. There are two distinct layers of ozone present in the Earth’s atmosphere. The troposphere, the layer closest to the earth, contains “evil” ozone. Tropospheric ozone is a toxic pollutant that develops as different substances produced by humans are changed by sunlight.
What is the process of ozone depletion?
They kill ozone molecules as chlorine and bromine atoms come into contact with ozone in the stratosphere. About 100,000 ozone molecules will be killed by one chlorine atom before it is eliminated from the stratosphere. More rapidly than normally produced, ozone can be lost.
How the ozone layer is healing?
According to a recent report, the ozone layer is beginning to regenerate and has the ability to fully restore. Published in Nature, a research paper heralds a rare achievement in repairing environmental damage and shows that concerted global action can make a difference.
Why is ozone layer important for us?
In the stratosphere (a layer of the atmosphere between 10 and 40 km above us), much of the ozone remains where it serves as a barrier to protect the surface of the Earth from the sun’s damaging ultraviolet radiation. We would be more vulnerable to skin cancer, cataracts and compromised immune systems if this protection were reduced.
To know more about the ozone layer and its depletion , sign up with BYJU’S and download BYJU’S – The Learning App.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Nice and help full app
Click here to learn more about Ozone Questions .
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- CBSE Class 10th
- CBSE Class 12th
- UP Board 10th
- UP Board 12th
- Bihar Board 10th
- Bihar Board 12th
Top Schools
- Top Schools in India
- Top Schools in Delhi
- Top Schools in Mumbai
- Top Schools in Chennai
- Top Schools in Hyderabad
- Top Schools in Kolkata
- Top Schools in Pune
- Top Schools in Bangalore
Products & Resources
- JEE Main Knockout April
- Free Sample Papers
- Free Ebooks
- RD Sharma Solutions
- Navodaya Vidyalaya Admission 2024-25
NCERT Study Material
- NCERT Notes
- NCERT Books
- NCERT Syllabus
- NCERT Solutions
- NCERT Solutions for Class 12
- NCERT Solutions for Class 11
- NCERT solutions for Class 10
- JEE Main Exam
- JEE Advanced Exam
- BITSAT Exam
- View All Engineering Exams
- Colleges Accepting B.Tech Applications
- Top Engineering Colleges in India
- Engineering Colleges in India
- Engineering Colleges in Tamil Nadu
- Engineering Colleges Accepting JEE Main
- Top IITs in India
- Top NITs in India
- Top IIITs in India
- JEE Main College Predictor
- JEE Main Rank Predictor
- MHT CET College Predictor
- AP EAMCET College Predictor
- GATE College Predictor
- KCET College Predictor
- JEE Advanced College Predictor
- View All College Predictors
- JEE Advanced Cutoff
- JEE Main Cutoff
- GATE Registration 2025
- JEE Main Syllabus 2025
- Download E-Books and Sample Papers
- Compare Colleges
- B.Tech College Applications
- JEE Main Question Papers
- View All Management Exams
Colleges & Courses
- Top MBA Colleges in India
- MBA College Admissions
- MBA Colleges in India
- Top IIMs Colleges in India
- Top Online MBA Colleges in India
- MBA Colleges Accepting XAT Score
- BBA Colleges in India
- XAT College Predictor 2025
- SNAP College Predictor
- NMAT College Predictor
- MAT College Predictor 2024
- CMAT College Predictor 2025
- CAT Percentile Predictor 2024
- CAT 2024 College Predictor
- Top MBA Entrance Exams 2024
- SNAP Registration
- GD Topics for MBA
- CAT 2024 Admit Card
- Download Helpful Ebooks
- List of Popular Branches
- QnA - Get answers to your doubts
- IIM Fees Structure
- AIIMS Nursing
- Top Medical Colleges in India
- Top Medical Colleges in India accepting NEET Score
- Medical Colleges accepting NEET
- List of Medical Colleges in India
- List of AIIMS Colleges In India
- Medical Colleges in Maharashtra
- Medical Colleges in India Accepting NEET PG
- NEET College Predictor
- NEET PG College Predictor
- NEET MDS College Predictor
- NEET Rank Predictor
- DNB PDCET College Predictor
- NEET Syllabus 2025
- NEET Study Material 2024
- NEET Cut off
- NEET Exam Date 2025
- Download Helpful E-books
- Colleges Accepting Admissions
- Top Law Colleges in India
- Law College Accepting CLAT Score
- List of Law Colleges in India
- Top Law Colleges in Delhi
- Top NLUs Colleges in India
- Top Law Colleges in Chandigarh
- Top Law Collages in Lucknow

Predictors & E-Books
- CLAT College Predictor
- MHCET Law ( 5 Year L.L.B) College Predictor
- AILET College Predictor
- Sample Papers
- Compare Law Collages
- Careers360 Youtube Channel
- CLAT Syllabus 2025
- Free CLAT Practice Test
- NID DAT Exam
- Pearl Academy Exam
Predictors & Articles
- NIFT College Predictor
- UCEED College Predictor
- NID DAT College Predictor
- NID DAT 2025
- NID DAT Syllabus 2025
- Design Colleges in India
- Top NIFT Colleges in India
- Fashion Design Colleges in India
- Top Interior Design Colleges in India
- Top Graphic Designing Colleges in India
- Fashion Design Colleges in Delhi
- Fashion Design Colleges in Mumbai
- Top Interior Design Colleges in Bangalore
- NIFT Cutoff
- NIFT Fees Structure
- NIFT Syllabus 2025
- Free Design E-books
- List of Branches
- Careers360 Youtube channel
- IPU CET BJMC 2024
- JMI Mass Communication Entrance Exam 2024
- IIMC Entrance Exam 2024
- MICAT Exam 2025
- Media & Journalism colleges in Delhi
- Media & Journalism colleges in Bangalore
- Media & Journalism colleges in Mumbai
- List of Media & Journalism Colleges in India
- CA Intermediate
- CA Foundation
- CS Executive
- CS Professional
- Difference between CA and CS
- Difference between CA and CMA
- CA Full form
- CMA Full form
- CS Full form
- CA Salary In India
Top Courses & Careers
- Bachelor of Commerce (B.Com)
- Master of Commerce (M.Com)
- Company Secretary
- Cost Accountant
- Charted Accountant
- Credit Manager
- Financial Advisor
- Top Commerce Colleges in India
- Top Government Commerce Colleges in India
- Top Private Commerce Colleges in India
- Top M.Com Colleges in Mumbai
- Top B.Com Colleges in India
- IT Colleges in Tamil Nadu
- IT Colleges in Uttar Pradesh
- MCA Colleges in India
- BCA Colleges in India
Quick Links
- Information Technology Courses
- Programming Courses
- Web Development Courses
- Data Analytics Courses
- Big Data Analytics Courses
- RUHS Pharmacy Admission Test
- Top Pharmacy Colleges in India
- Pharmacy Colleges in Pune
- Pharmacy Colleges in Mumbai
- Colleges Accepting GPAT Score
- Pharmacy Colleges in Lucknow
- List of Pharmacy Colleges in Nagpur
- GPAT Result
- GPAT 2024 Admit Card
- GPAT Question Papers
- NCHMCT JEE 2025
- Mah BHMCT CET
- Top Hotel Management Colleges in Delhi
- Top Hotel Management Colleges in Hyderabad
- Top Hotel Management Colleges in Mumbai
- Top Hotel Management Colleges in Tamil Nadu
- Top Hotel Management Colleges in Maharashtra
- B.Sc Hotel Management
- Hotel Management
- Diploma in Hotel Management and Catering Technology
Diploma Colleges
- Top Diploma Colleges in Maharashtra
- UPSC IAS 2024
- SSC CGL 2024
- IBPS RRB 2024
- Previous Year Sample Papers
- Free Competition E-books
- Sarkari Result
- QnA- Get your doubts answered
- UPSC Previous Year Sample Papers
- CTET Previous Year Sample Papers
- SBI Clerk Previous Year Sample Papers
- NDA Previous Year Sample Papers
Upcoming Events
- NDA 2 Admit card 2024
- SSC CGL Admit card 2024
- CDS 2 Admit card 2024
- UGC NET Admit card 2024
- HP TET Result 2024
- SSC CHSL Result 2024
- UPTET Notification 2024
- SBI PO Notification 2024
Other Exams
- SSC CHSL 2024
- UP PCS 2024
- UGC NET 2024
- RRB NTPC 2024
- IBPS PO 2024
- IBPS Clerk 2024
- IBPS SO 2024
- Top University in USA
- Top University in Canada
- Top University in Ireland
- Top Universities in UK
- Top Universities in Australia
- Best MBA Colleges in Abroad
- Business Management Studies Colleges
Top Countries
- Study in USA
- Study in UK
- Study in Canada
- Study in Australia
- Study in Ireland
- Study in Germany
- Study in China
- Study in Europe
Student Visas
- Student Visa Canada
- Student Visa UK
- Student Visa USA
- Student Visa Australia
- Student Visa Germany
- Student Visa New Zealand
- Student Visa Ireland
- CUET PG 2025
- UP B.Ed JEE 2024
- TS EDCET Exam
- IIT JAM 2025
- AP PGCET Exam
- Universities in India
- Top Universities in India 2024
- Top Colleges in India
- Top Universities in Uttar Pradesh 2024
- Top Universities in Bihar
- Top Universities in Madhya Pradesh 2024
- Top Universities in Tamil Nadu 2024
- Central Universities in India
- CUET DU Cut off 2024
- IGNOU Date Sheet 2024
- CUET DU CSAS Portal 2024
- CUET 2025 Syllabus
- CUET PG Syllabus 2025
- CUET Participating Universities 2025
- CUET Previous Year Question Paper
- IGNOU Result 2024
- E-Books and Sample Papers
- CUET College Predictor 2024
- CUET Exam Date 2025
- CUET Cut Off 2024
- NIRF Ranking 2024
- IGNOU Exam Form 2024
- CUET Syllabus
- CUET Counselling 2025
Engineering Preparation
- Knockout JEE Main 2024
- Test Series JEE Main 2024
- JEE Main 2024 Rank Booster
Medical Preparation
- Knockout NEET 2024
- Test Series NEET 2024
- Rank Booster NEET 2024
Online Courses
- JEE Main One Month Course
- NEET One Month Course
- IBSAT Free Mock Tests
- IIT JEE Foundation Course
- Knockout BITSAT 2024
- Career Guidance Tool
Top Streams
- IT & Software Certification Courses
- Engineering and Architecture Certification Courses
- Programming And Development Certification Courses
- Business and Management Certification Courses
- Marketing Certification Courses
- Health and Fitness Certification Courses
- Design Certification Courses
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
- UG Degree Courses
- PG Degree Courses
- Short Term Courses
- Free Courses
- Online Degrees and Diplomas
- Compare Courses
Top Providers
- Coursera Courses
- Udemy Courses
- Edx Courses
- Swayam Courses
- upGrad Courses
- Simplilearn Courses
- Great Learning Courses
Ozone Day Speech - International Ozone Day (September 16)
Every year on September 16, International Ozone Day is celebrated to draw attention to the harmful effects of ozone depletion and to highlight the importance of the ozone layer. This day is a reminder of the need to protect the ozone layer. It also highlights the need for more effective action. Several reports claim that the already affected ozone layer will continue to be affected for 50 to 100 years or more, which could worsen the human condition in the future. There are many ways to celebrate World Ozone Day, and one of them is to educate people about the benefits of protecting the ozone layer.
10 Lines on Ozone Day Speech
Ozone day speech in 100 words, ozone day speech in 200 words, short speech on ozone day, long speech on ozone day.

Every year on September 16, we monitor the world's ozone layer and disseminate information about it.
The United Nations General Assembly declared this day as World Ozone Day.
Ozone is made up of three oxygen atoms and exists in the stratosphere.
It protects us from the sun's ultraviolet rays, which can be fatal to live organisms.
These rays can cause serious injuries such as cancer if the ozone is weakened.
However, scientists have found several holes in it, which is not a good sign.
This is due to environmental deterioration and rising temperatures.
Human activities that harm the environment should be monitored regularly.
Increased vehicle use, heating and cooling, and deforestation, even over short distances, are just a few factors contributing to ozone depletion.
Planting trees and using sustainable products protects the ozone layer.
Every year, we celebrate the World Ozone Day on September 16. This year the theme for World Ozone Day 2024 is "Ozone for Life: 35 years of global cooperation". The goal of this theme is to highlight the collective efforts and commitment of the world to protect the ozone layer over the past 35 years.
The Ozone layer acts as a protective shield that absorbs the harmful ultraviolet (UV) radiation of the sun. Without the ozone layer, the Earth will be directly exposed to UV radiation that will lead to a rise in skin cancer, cataracts and other serious health issues. It will also lead to damaging the ecosystems and marine life on Earth. Hence, is it significant to celebrate World Ozone Day. It helps bring attention to the importance of the ozone layer and the need to preserve it.
The World Ozone Day is celebrated globally on September 16. This year as well, World Ozone Day will be celebrated and the theme for this year is "Ozone for Life: 35 years of global cooperation". The World Ozone Day was established by the United Nations General Assembly. The aim of celebrating this day is to raise global awareness about the importance of the ozone layer and protect it. September 16 commemorates the signing of the Montreal Protocol in 1987. It is a landmark international treaty that was aimed at phasing out ozone-depleting chemicals, such as chlorofluorocarbons (CFCs).
The depletion of the Ozone layer can cause a threat to life on Earth. Without the Ozone layer harmful ultraviolet (UV) radiation will be directly exposed to Earth's surface that will lead to health risk such as skin cancer and environmental harm. Hence, on World Ozone Day, we highlight the success of the global initiatives that ensure a healthier and more sustainable future for all living beings on Earth. And the theme of World Ozone Day 2024 highlights exactly that, the collective efforts and commitment of the world to protect the ozone layer over the past 35 years.
Ozone Day is a day established by the United Nations General Assembly to raise awareness of the importance of the ozone layer. Also on this day, they talk about harmful substances that threaten the condition of the ozone layer. The United Nations General Assembly designated September 16 as Ozone Day because it signed the Montreal Protocol for the Protection of the Ozone Layer in 1987.
Every year, World Ozone Day for the preservation of the ozone layer is celebrated with a unique theme. This theme is presented by the United Nations and is the basis of numerous events, debates and celebrations around the world. This year's theme for ozone day 2024 is "Ozone for Life: 35 years of global cooperation".
The theme of World Ozone Day 2024 represents the collective efforts and commitment of the world to protect the ozone layer over the past 35 years.
Ozone Layer Day is considered an important annual event. Many prominent global organisations gathered on this day to talk about the need to protect the ozone layer. Depletion of the ozone layer poses a serious threat to existing life on Earth. As such, events such as Ozone Day highlight global cooperation to eliminate substances and human activities that threaten the ozone layer.
The main purpose of celebrating World Day for the Preservation of the Ozone Layer is to spread awareness to people about the depletion of the ozone layer and to find possible solutions to save it. First, it is important to understand what the ozone layer is and why it is so important to human life. It contains high concentrations of ozone (O3) in different parts of the atmosphere. The ozone layer is very important for protecting human life because it absorbs most of the ultraviolet radiation that reaches the Earth from the sun. Plants cannot live or grow in strong UV light, and plankton cannot be food for most marine life. A weakening of the ozone layer will make people more vulnerable to skin cancer, cataracts and compromised immune systems.
However, there is an important point we need to keep in mind. Ozone can either protect the Earth or harm it. For example, if there is an ozone layer in the atmosphere's stratosphere, it acts as a protective barrier. However, when ozone is present in the troposphere (about 10 km above the Earth's surface), it is harmful. In the troposphere, it is a pollutant that can damage lung tissue and plants. Most of the ozone remains in the stratosphere, where it acts as a shield and protects the earth's surface from harmful ultraviolet rays from the sun. In 1994, the United Nations General Assembly declared September 16 as World Ozone Layer Preservation Day to protect the ozone layer. In 1987, the Montreal Protocol on substances that use up the Ozone Layer turned into signed, a global conference used to shield the ozone layer. It is designed to protect the Earth's ozone layer by stopping the production and import of ozone-depleting substances and reducing their concentrations in the atmosphere.
The Montreal Protocol is considered the most successful environmental agreement signed in 1987. It established a mandatory schedule for phasing out ozone-depleting substances.
Governments of all countries take necessary steps to protect the ozone layer, but did you know that they can also take several other steps to protect the ozone layer? For example, it is said to minimise vehicle use. You may want to avoid using some cleaning products that are harmful to both you and the environment. What many people don't realise is that many cleaning products on the market today also contain caustics and solvents, and these harmful substances can be replaced with products that are not environmentally toxic, such as bicarbonate or vinegar. There are a few more things we can do to protect the ozone layer.
As responsible citizens, all we can do is educate people on this serious topic. We should share them with our friends, family and followers. It's a wonderful and inspiring job for us to do. One person doing something and another person doing it all add up to a big change.
Ozone Day is celebrated to commemorate the signing of the Montreal Protocol. This milestone led to a collaboration between 24 countries to protect the ozone layer and fight substances that threaten it. On September 16, 2009, the Montreal Protocol became one of the first treaties to receive universal ratification.
Applications for Admissions are open.

VMC VIQ Scholarship Test
Register for Vidyamandir Intellect Quest. Get Scholarship and Cash Rewards.

JEE Main Important Physics formulas
As per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

JEE Main Important Chemistry formulas
As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

TOEFL ® Registrations 2024
Accepted by more than 11,000 universities in over 150 countries worldwide

Pearson | PTE
Register now for PTE & Unlock 20% OFF : Use promo code: 'C360SPL20'. Valid till 15th NOV'24! Trusted by 3,500+ universities globally

JEE Main high scoring chapters and topics
As per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
Download Careers360 App
All this at the convenience of your phone.
Regular Exam Updates
Best College Recommendations
College & Rank predictors
Detailed Books and Sample Papers
Question and Answers
Scan and download the app
- ENVIRONMENT
What is the ozone layer, and why does it matter?
Human activity has damaged this protective layer of the stratosphere, but scientists say the ozone layer is on track for recovery.
Earth's ozone layer, an early symbol of global environmental degradation, is improving and on track to recover by the middle of the 21st century.
Over the past 30 years, humans have successfully phased out many of the chemicals that harm the ozone layer , the atmospheric shield that sits in the stratosphere about nine to 18 miles (15 to 30 kilometers) above Earth's surface.
Atmospheric ozone absorbs ultraviolet (UV) radiation from the sun, particularly harmful UVB-type rays. Exposure to UVB radiation is linked with increased risk of skin cancer and cataracts, as well as damage to plants and marine ecosystems. Atmospheric ozone is sometimes labeled as the "good" ozone, because of its protective role, and shouldn't be confused with tropospheric, or ground-level, "bad" ozone, a key component of air pollution that is linked with respiratory disease.
( See where air pollution is lethal. )
Ozone (O3) is a highly reactive gas whose molecules are comprised of three oxygen atoms. Its concentration in the atmosphere naturally fluctuates depending on seasons and latitudes, but it was generally stable when global measurements began in 1957 .
Groundbreaking research in the 1970s and 1980s revealed signs of trouble.
Ozone threats and 'the hole'
In 1974, Mario Molina and Sherwood Rowland, two chemists at the University of California, Irvine, published an article in the journal Nature detailing threats to the ozone layer from chlorofluorocarbon (CFC) gases. At the time, CFCs were commonly used in aerosol sprays and as coolants in many refrigerators. As they reach the stratosphere, the sun's UV rays break CFCs down into substances such as chlorine.
This groundbreaking research—for which they were awarded the 1995 Nobel Prize in chemistry —concluded that the atmosphere had a “finite capacity for absorbing chlorine” atoms in the stratosphere.
One atom of chlorine can destroy more than 100,000 ozone molecules, according to the U.S. Environmental Protection Agency , eradicating ozone much more quickly than it can be replaced.
Molina and Rowland’s study was validated in 1985, when a team of English scientists found a hole in the ozone layer over Antarctica that was later linked to CFCs. The "hole" is actually an area of the stratosphere with extremely low concentrations of ozone that reoccurs every year at the beginning of the Southern Hemisphere spring (August to October).
At the North Pole, a degraded ozone layer is responsible for the Arctic's rapid rate of warming, according to a 2020 study published in Nature Climate Change . CFCs are a more potent greenhouse gas than carbon dioxide, the most abundant planet-warming gas.

Aerosol from cans sometimes contains ozone-depleting substances called chlorofluorocarbons, or CFCs.
The ozone layer’s status today
In a report released in early 2023 , scientists keeping track of the ozone layer noted that Earth's atmosphere is recovering. The ozone layer will be restored to its 1980 condition—before the ozone hole emerged—by 2040. More persistent ozone holes over the Arctic and Antarctica should recover by 2045 and 2066, respectively.
This progress is thanks to the Montreal Protocol on Substances That Deplete the Ozone Layer , a landmark agreement signed by 197 UN member countries in 1987 to phase out ozone-depleting substances. Without the pact, the EPA estimates the U.S. would have seen an additional 280 million cases of skin cancer, 1.5 million skin cancer deaths, and 45 million cataracts—and the world would be at least 25 percent hotter.
( Read more about how climate change is a threat to human health. )
Nearly all the ozone-destroying chemicals banned by the Montreal Protocol have been phased out, but some harmful gases are still used. Hydrochlorofluorocarbons (HCFCs), transitional substitutes that are less damaging but still harmful to ozone, are still in use in some countries. HCFCs are also powerful greenhouse gases that trap heat and contribute to climate change .
Though HFCs represent a small fraction of emissions compared with carbon dioxide and other greenhouse gases , their planet-warming effect prompted an addition to the Montreal Protocol, the Kigali Amendment , in 2016. The amendment, which came into force in January 2019, aims to slash the use of HFCs by more than 80 percent over the next three decades.
In the meantime, companies and scientists are working on climate-friendly alternatives, including new coolants and technologies that reduce or eliminate dependence on chemicals altogether.
YEAR-LONG ADVENTURE for every explorer on your list
Related topics.
- AIR POLLUTION
- ENVIRONMENT AND CONSERVATION
- CLIMATE CHANGE
You May Also Like

Air pollution, explained

Why deforestation matters—and what we can do to stop it

These breathtaking natural wonders no longer exist

Another weapon to fight climate change? Put carbon back where we found it

Apollo moon rocks reveal secrets of the moon’s thin ‘atmosphere’
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved

IMAGES
VIDEO
COMMENTS
Ozone layer consumption is the diminishing of the ozone layer present in the upper air. This happens when the chlorine and bromine iotas in the environment interact with ozone and crush the ozone atoms. …
Get a detailed Ozone Day essay in English. Explore the importance, history, and global significance of World Ozone Day. Learn why protecting the ozone layer is crucial for our planet.
Long World Ozone Day Essay in English. The ozone layer, often described as Earth's sunscreen, is an invisible yet essential shield that envelops our planet. Composed of ozone molecules, this layer resides in the …
The stratosphere includes the zone commonly called the ‘ozone layer’. It plays a crucial role in keeping the planet habitable by absorbing potentially dangerous ultraviolet (UV-B) radiation from the sun. Before its …
The ozone layer is a region of Earth’s stratosphere containing relatively high ozone concentration (O3) molecules. The ozone layer plays a significant role in protecting life on Earth by absorbing and blocking a …
Read About The Importance, Preparation, Structure, And Properties Of The Ozone Layer. Understand The Reasons For Ozone Layer Depletion and how it can be counteracted
Explore our free top-notch 'Ozone Layer' essay examples for insights and inspiration. Craft your own paper with our comprehensive database.
10 Lines on Ozone Day Speech. Every year on September 16, we monitor the world's ozone layer and disseminate information about it. The United Nations General Assembly declared this day as World Ozone Day. Ozone is …
The ozone layer is a layer found in the Earth's stratosphere at a height of 10 km, holding a great concentration of ozone. A molecule of ozone (O3) contains three oxygen atoms …
Earth's ozone layer, an early symbol of global environmental degradation, is improving and on track to recover by the middle of the 21st century.