Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence
Affiliations.
- 1 Department of Pharmacy, School of Medicine, University of Naples Federico II, Via Montesano 49, 80131 Naples, Italy.
- 2 Department of Experimental Medicine, Tor Vergata University of Rome, Via Montpellier 1, 00133 Rome, Italy.
- 3 Department of Experimental Medicine, Tor Vergata University of Rome, Via Montpellier 1, 00133 Rome, Italy. [email protected].
- PMID: 31277273
- PMCID: PMC6682953
- DOI: 10.3390/nu11071514
Breast cancer (BC) is the second most common cancer worldwide and the most commonly occurring malignancy in women. There is growing evidence that lifestyle factors, including diet, body weight and physical activity, may be associated with higher BC risk. However, the effect of dietary factors on BC recurrence and mortality is not clearly understood. Here, we provide an overview of the current evidence obtained from the PubMed databases in the last decade, assessing dietary patterns, as well as the consumption of specific food-stuffs/food-nutrients, in relation to BC incidence, recurrence and survival. Data from the published literature suggest that a healthy dietary pattern characterized by high intake of unrefined cereals, vegetables, fruit, nuts and olive oil, and a moderate/low consumption of saturated fatty acids and red meat, might improve overall survival after diagnosis of BC. BC patients undergoing chemotherapy and/or radiotherapy experience a variety of symptoms that worsen patient quality of life. Studies investigating nutritional interventions during BC treatment have shown that nutritional counselling and supplementation with some dietary constituents, such as EPA and/or DHA, might be useful in limiting drug-induced side effects, as well as in enhancing therapeutic efficacy. Therefore, nutritional intervention in BC patients may be considered an integral part of the multimodal therapeutic approach. However, further research utilizing dietary interventions in large clinical trials is required to definitively establish effective interventions in these patients, to improve long-term survival and quality of life.
Keywords: breast cancer; diet; food; nutrients; prevention.

Publication types
- Breast Neoplasms / diet therapy*
- Breast Neoplasms / epidemiology
- Breast Neoplasms / prevention & control*
- Diet / adverse effects*
- Diet, Healthy*
- Middle Aged
- Nutritional Status
- Nutritive Value*
- Protective Factors
- Recommended Dietary Allowances
- Risk Assessment
- Risk Factors
- Risk Reduction Behavior*
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Breast Cancer Treatments: Updates and New Challenges
Anna burguin, caroline diorio, francine durocher.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected] ; Tel.: +1-418-525-4444 (ext. 48508)
Received 2021 Jun 30; Accepted 2021 Aug 16; Collection date 2021 Aug.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Breast cancer (BC) is the most frequent cancer diagnosed in women worldwide. This heterogeneous disease can be classified into four molecular subtypes (luminal A, luminal B, HER2 and triple-negative breast cancer (TNBC)) according to the expression of the estrogen receptor (ER) and the progesterone receptor (PR), and the overexpression of the human epidermal growth factor receptor 2 (HER2). Current BC treatments target these receptors (endocrine and anti-HER2 therapies) as a personalized treatment. Along with chemotherapy and radiotherapy, these therapies can have severe adverse effects and patients can develop resistance to these agents. Moreover, TNBC do not have standardized treatments. Hence, a deeper understanding of the development of new treatments that are more specific and effective in treating each BC subgroup is key. New approaches have recently emerged such as immunotherapy, conjugated antibodies, and targeting other metabolic pathways. This review summarizes current BC treatments and explores the new treatment strategies from a personalized therapy perspective and the resulting challenges.
Keywords: breast cancer, personalized therapies, molecular subtypes, breast cancer treatment, luminal, HER2, TNBC
1. Introduction
Breast cancer (BC) is the most frequent cancer and the second cause of death by cancer in women worldwide. According to Cancer Statistics 2020, BC represents 30% of female cancers with 276,480 estimated new cases and more than 42,000 estimated deaths in 2020 [ 1 ].
Invasive BC can be divided into four principal molecular subtypes by immunohistological technique based on the expression of the estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) [ 2 ]. Luminal A BC (ER+ and/or PR+, and HER2-) represents around 60% of BC and is associated with a good prognosis [ 3 ]. Luminal B BC (ER+ and/or PR+, and HER2+) represents 30% of BC and is associated with high ki67 (>14%), a proliferation marker, and a poor prognosis [ 4 ]. HER2 BC (ER-, PR-, and HER2+) represents 10% of BC and is also associated with a poor prognosis [ 5 ]. Lastly, triple-negative BC (TNBC) (ER-, PR-, and HER2-) represents 15–20% of BC and is associated with more aggressivity and worse prognosis compared to other BC molecular subtypes and often occurs in younger women [ 6 ]. Characteristics of BC by molecular subtypes are described in Figure 1 .
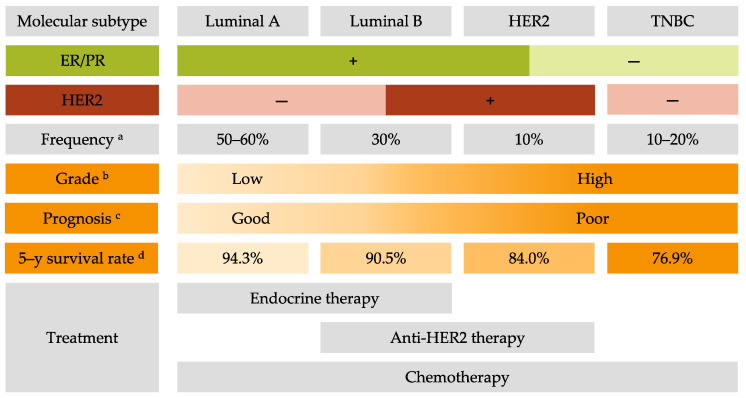
Characteristics of breast cancer molecular subtypes. ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; TNBC: triple-negative breast cancer. a . Frequency derived from Al-thoubaity et al. [ 12 ] and Hergueta-Redondo et al. [ 13 ]. b . Grade derived from Engstrom et al. [ 14 ]. c . Prognosis derived from Hennigs et al. [ 15 ] and Fragomeni et al. [ 16 ]. d . The 5–year survival rate derived from the latest survival statistics of SEER [ 7 ].
The 5-year relative BC-specific survival rate of BC is encouraging with 90.3% for all subtypes and stages. However, for metastatic BC the 5-year relative cancer-specific survival rate is still low: 29% regardless of subtype and can drop to 12% for metastatic TNBC [ 7 ]. This clearly indicates that strategies of treatment for metastatic BC patients are not effective enough to ensure a good survival rate. Thus, it is crucial to find new solutions for the treatment of metastatic BC and especially TNBC.
Treatment choice is based on the grade, stage, and BC molecular subtype to have the most personalized, safe, and efficient therapy. The grade describes the appearance of tumor cells compared to normal cells. It includes tubule differentiation, nuclear pleomorphism, and the mitotic count [ 8 ]. The stage is used to classify the extent of cancer in the body and is defined using the TNM system comprising tumor size, lymph node status, and the presence of metastases [ 9 ]. For non-metastatic BC, the strategic therapy involves removing the tumor by complete or breast-conserving surgery with preoperative (neoadjuvant) or postoperative (adjuvant) radiotherapy and systemic therapy including chemotherapy, and targeted therapy. Targeted therapy comprises endocrine therapy for hormone receptor-positive (HR+) BC and anti-HER2 therapy for HER2+ BC. Unfortunately, there is no available targeted therapy for the TNBC subtype. For metastatic BC the priority is to contain tumor spread as this type of BC remains incurable. The same systemic therapies are used to treat metastatic BC [ 10 ].
Challenges in the treatment of BC including dealing with treatment resistance and recurrence. Indeed, 30% of early-stage BC have recurrent disease, mostly metastases [ 11 ]. Thus, it is crucial to develop new strategic therapies to treat each BC subgroup effectively.
This review will summarize current treatments for invasive BC, the underlying resistance mechanisms and explore new treatment strategies focusing on personalized therapy and the resulting challenges.
2. Common Treatments for All Breast Cancer Subtypes
In addition to surgery, radiotherapy and chemotherapy are used routinely to treat all BC subtypes [ 17 ].
2.1. Surgery
The most standard breast surgery approaches are either total excision of the breast (mastectomy), usually followed by breast reconstruction, or breast-conserving surgery (lumpectomy). Lumpectomy entails the excision of the breast tumor with a margin of surrounding normal tissue. The recommended margins status is defined as “no ink on tumor”, meaning no remaining tumor cells at the tissue edge [ 18 ]. Studies show that total mastectomy and lumpectomy plus irradiation are equivalent regarding relapse-free and overall survival (OS) [ 19 ]. Contraindications for breast-conserving surgery include the presence of diffuse microcalcifications (suspicious or malignant-appearing), disease that cannot be incorporated by local excision with satisfactory cosmetic result, and ATM (ataxia-telangiesctasia mutated) mutation (biallelic inactivation) [ 18 ].
The surgery to remove axillary lymph nodes is useful to determine cancerous cell spread and for therapeutic purposes. For instance, axillary lymph node dissection (ALND) can improve survival rated by removing remaining tumor cells. ALND used to be the goal standard for removing positive lymph nodes. However, clinical trials showed that sentinel lymph node biopsy (SLNB) had the same effect as ALND regarding disease-free survival (DFS) and OS [ 20 ]. Other clinical trials demonstrated that ALND was not necessary for all patients with positive lymph nodes. Moreover, most patients who receive radiation and systemic treatment after SLNB have negative lymph nodes as these treatments are sufficient in eliminating residual tumor cells [ 21 ].
2.2. Radiotherapy
Radiation therapy has been used to treat cancer since Röngten discovered the X-ray in 1895 [ 22 ]. High-energy radiations are applied to the whole breast or a portion of the breast (after breast-conservative surgery), chest wall (after mastectomy), and regional lymph nodes [ 23 ]. A meta-analysis showed that radiation following conservative surgery offered more benefits to patients with higher-risk BC while patients with small, low-grade tumors could forego radiation therapy [ 24 ]. Postmastectomy radiation to the chest wall in patients with positive lymph nodes is associated with decreased recurrence risk and BC mortality compared to patients with negative lymph nodes [ 25 ]. A radiation boost to the regional node radiation treatment can be incorporated after mastectomy for patients at higher risk for recurrence [ 26 ]. This additional radiation boost to regional nodes following mastectomy is associated with improved (DFS) but is also associated with an increase in radiation toxicities such as pneumonitis and lymphedema [ 27 ]. Radiotherapy can be administered concurrently with personalized therapy (anti-HER2 therapy or endocrine therapy).
As one of the major side effects of radiotherapy is cardiotoxicity, it is critical to minimize exposure to the heart and lungs [ 28 ]. Additional techniques can be used to reduce the radiation exposure to the heart, lungs, and normal tissue such as prone positioning, respiratory control, or intensity-modulated radiotherapy [ 29 ].
Advanced invasive BC can exhibit radiation therapy resistance [ 30 ]. The hypoxic tumor microenvironment, which lacks oxygen, leads to increased cell proliferation, apoptosis resistance, and radiotherapy resistance [ 31 ]. The major player of this resistance is the HIF-1α (hypoxia-inducible factor 1 alpha) protein [ 32 ]. Indeed, HIF-1α overexpression is caused by low oxygen levels within the microenvironment and promotes the maintenance of hypoxia by allowing tumoral cells to survive in a hypoxic microenvironment [ 33 , 34 , 35 ]. Cancer stem cells (CSC) could also have a role in radiation therapy resistance [ 36 ]. CSC can self-renew and initiate subpopulations of differential progeny, and a hypoxic microenvironment is ideal for CSC survival and proliferation [ 37 , 38 ].
Radiation therapy is used to treat all BC subtypes, but its implication is more important for TNBC, as there is no personalized therapy for this subtype. It has been shown that radiotherapy benefits TNBC patients both after conserving surgery and mastectomy [ 39 ].
2.3. Chemotherapy
BC chemotherapy comprises several families of cytotoxic drugs, including alkylating agents, antimetabolites and tubulin inhibitors [ 40 ]. Cyclophosphamide is a nitrogen mustard alkylating agent causing breakage of the DNA strands [ 41 ]. The mechanism of action for anthracyclines (doxorubicin, daunorubicin, epirubicin, and idarubicin) includes DNA intercalation, thereby inhibiting macromolecular biosynthesis [ 42 ]. Taxanes, including docetaxel and paclitaxel, bind to microtubules and prevent their disassembly, leading to cell cycle arrest and apoptosis [ 43 ].
Chemotherapy can be administered in the neoadjuvant or adjuvant setting and for metastatic BC treatment.
2.3.1. Neoadjuvant Chemotherapy (NAC)
Neoadjuvant chemotherapy was initially administered for non-metastatic but inoperable BC, defined as unreachable tumors [ 44 ]. Then, chemotherapy was used before the surgery for operable tumors to facilitate breast conservation [ 45 ].
Studies demonstrated that chemotherapy administered before surgery is as effective as administered after surgery [ 46 , 47 , 48 ]. The NSABP-B-18 trial compared the effects of doxorubicin and cyclophosphamide administered either postoperatively or preoperatively. This trial showed that NAC reduces the rate of axillary metastases in node-negative BC patients [ 48 ].
Some patients fail to achieve pathologic complete response after a full course of NAC. Unfortunately, there is no consensus regarding the treatment strategy to follow for patients with residual disease after surgery [ 49 , 50 ]. The BC subtype plays an important role in the response to NAC. Indeed, TNBC and HER2+ BC are more likely to be sensitive to chemotherapy. Hence, NAC is a good strategy to maximize pathologic complete response in these BC subtypes [ 45 ].
2.3.2. Adjuvant Chemotherapy
Adjuvant chemotherapy is administered to BC patients with lymph nodes metastases or a high risk of recurrence [ 51 ]. The standard chemotherapy treatment comprises an anthracycline and a taxane. The two most common regimens are cyclophosphamide and doxorubicin for four cycles followed by paclitaxel for four cycles. Then patients are given the previous combination of therapies followed by either weekly paclitaxel for 12 weeks, or docetaxel every 3 weeks for four cycles [ 52 , 53 ].
Like neoadjuvant therapy, patients with HR-negative BC receive more benefits from adjuvant therapy (i.e., reduction of BC recurrence and mortality) than HR+ BC patients [ 54 ]. However, for patients with HR+, node-negative BC associated with a high Oncotype recurrence score (≥31), calculated from the expression of 16 BC-related genes and 5 reference genes, adjuvant chemotherapy reduces the risk of recurrence [ 55 ]. The TAILORx clinical trial showed that HR+ BC patients with a low Oncotype recurrence score do not benefit from chemotherapy alone [ 56 ].
According to the molecular BC subtype, chemotherapy can be administered with targeted therapies. Patients with HR+ BC should receive endocrine therapy after chemotherapy is completed, and HER2+ BC patients should receive trastuzumab combined with chemotherapy [ 57 ]. For TNBC patients, front-line therapy includes a combination of taxane and anthracycline [ 58 ].
One of the major drawbacks of chemotherapy is its side effects. The early side effects (0–6 months of treatment) involve fatigue, alopecia, cytopenia (reduction in the number of normal blood cells), muscle pain, neurocognitive dysfunction, and chemo-induced peripheral neuropathy. The chronic or late side effects (after 6 months of treatment) include cardiomyopathy, second cancers, early menopause, sterility, and psychosocial impacts [ 59 ].
As mentioned previously in this review, chemotherapy is composed of taxanes, anthracyclines and cyclophosphamide. Each of these molecules can lead to resistance in BC patients [ 60 ].
One mechanism of resistance is by overexpressing p-glycoprotein, an ATP-binding cassette (ABC) family member, which confers resistance to anthracycline and taxanes [ 61 ]. Breast cancer resistance protein (BCRP), another ABC family member, induces resistance to anthracycline but not taxanes when overexpressed [ 62 ]. Microtubule alterations can also lead to taxane resistance. The overexpression of β-tubulin III induces paclitaxel resistance [ 63 ]. Moreover, mutations in microtubule-associated proteins (MAPs) affect microtubule dynamics and improve taxane resistance [ 64 ]. Multiple enzymes are known to be involved in the cyclophosphamide detoxification, leading to its resistance. For example, aldehyde dehydrogenase upregulation detoxifies aldophosphamide a type of cyclophosphamide, and mutations in glutathione S-transferases, enzymes involved in drug-metabolizing conjugation reactions, can also affect cyclophosphamide detoxification [ 65 , 66 ].
Surgery, radiotherapy, and chemotherapy are complementary strategies in the treatment of BC patients. However, they are not sufficient to effectively treat all BC molecular subtypes, as they do not have the same response to radiotherapy or chemotherapy. Thus, personalized therapies are essential in the process for BC treatment.
3. Current Personalized Treatments for Breast Cancer: Strengths and Weaknesses
The current strategies of treatment are principally based on the tumor progression and BC molecular subtypes in order to offer the most personalized treatment for BC patients. The algorithm of BC treatment is represented in Figure 2 .
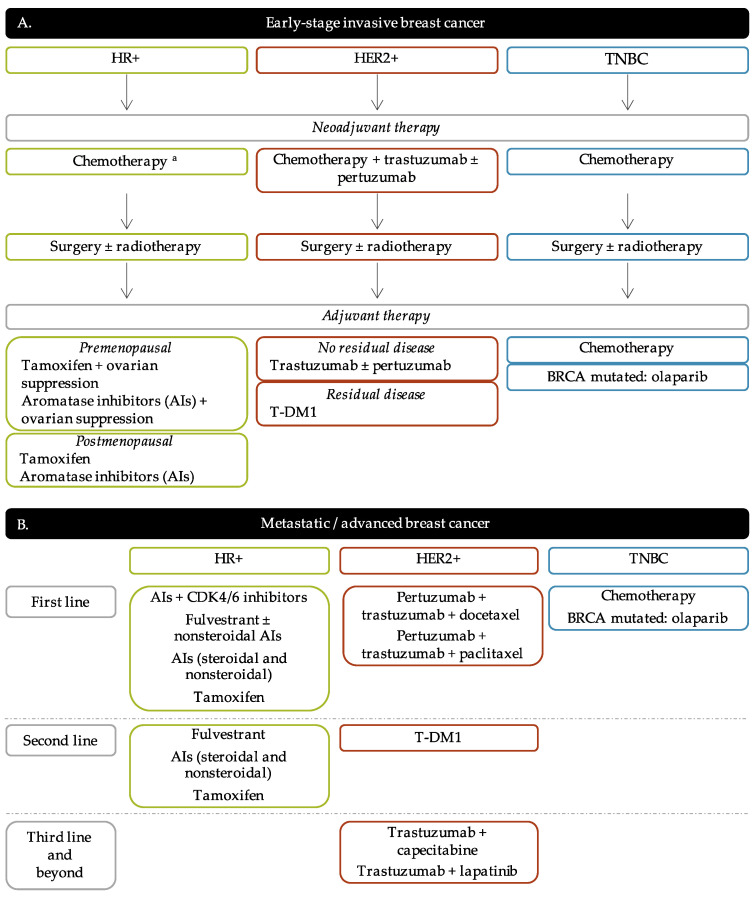
Breast cancer treatment flow diagram. ( A ). Early-stage breast cancer. ( B ). Metastatic/advanced breast cancer. a Neoadjuvant chemotherapy for HR+ BC patients is not systematic. It is mainly administered to luminal B BC patients and/or elder BC patients. HR+: hormone receptors positive; HER2+: human epidermal growth factor receptor 2 positive; TNBC: triple-negative breast cancer; AIs: aromatase inhibitors; T-DM1: trastuzumab-emtansine.
3.1. Endocrine Therapy
Endocrine therapy is the main strategy to treat HR positive invasive BC. The purpose of this therapy is to target the ER directly (selective estrogen receptors modulators and degraders) or the estrogen synthesis (aromatase inhibitors) [ 67 ]. The most common types of endocrine therapy are selective estrogen receptor modulators (SERMs), selective modulators estrogen receptor degraders (SERDs), and aromatase inhibitors (AIs) [ 68 ]. Endocrine therapy mechanism of action and resistance are described in Figure 3 .
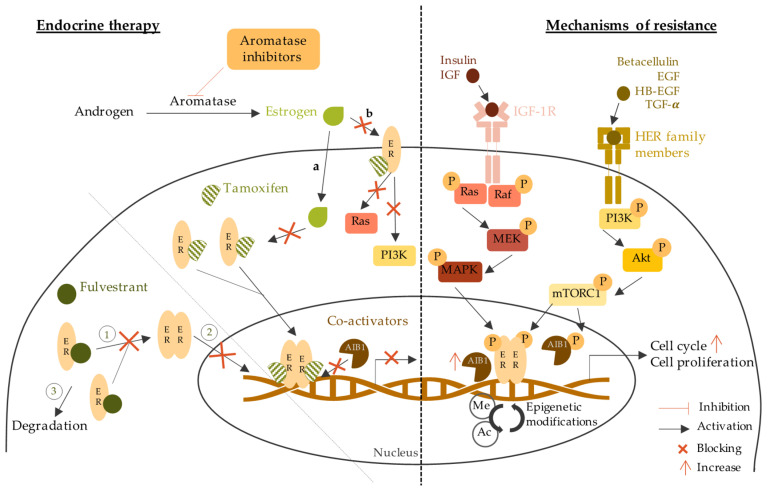
Endocrine therapy mechanisms of action and resistance. The left part of the figure shows the mechanism of endocrine therapy through aromatase inhibitors, tamoxifen, and fulvestrant. The right part of the figure describes the mechanisms of resistance to endocrine therapy through the epigenetic modifications, the increase of coactivators and cell cycle actors, and the activation of other signaling pathways. Estrogens can go through the plasma membrane by a. diffusion as they are small non-polar lipid soluble molecules; b. binding to membrane ER initiating the activation of Ras/Raf/MAPK and PI3K/Akt signaling pathways which are blocked by tamoxifen. 1: inhibition of ER dimerization; 2: blockage of nucleus access; 3: ER degradation. ER: estrogen receptor; AIB1: amplified in breast cancer 1; IGF-1R: insulin growth factor receptor 1; IGF: insulin growth factor; HER: human epidermal receptors; EGF: epidermal growth factor; HB-EGF: heparin-binding EGF-like growth factor; TGF-α: transforming growth factor alpha; MEK/MAPK: mitogen activated protein kinase; PI3K: phosphoinositide 3-kinase; mTOR: mammalian target of rapamycin; Me: methylation; Ac: acetylation.
3.1.1. Selective Estrogen Receptor Modulators (SERMs)
SERMs, such as tamoxifen, toremifene, bazedoxifene, and raloxifene, are antiestrogens that compete with estrogen by binding to the ER. This binding changes the conformation of the ER ligand-binding domain, and once ER is translocated to the nucleus, it blocks co-factor recruitment and subsequent genes transcription involved in cell cycle progression (cyclin D1), cell proliferation (like IGF-1), or cell migration (collagenase) [ 69 , 70 ].
The most used SERMs is tamoxifen, approved by the US Food and Drugs Administration (FDA) in 1977. It is an adjuvant therapy orally administered for 5 to 10 years according to tumor aggressivity. Tamoxifen adjuvant treatment reduces recurrence risk by 50% for the first 5 years and 30% for the next 5 years [ 71 ]. Tamoxifen is given to either premenopausal or postmenopausal patients. However, for high-risk premenopausal patients, adding ovarian suppression is more effective than tamoxifen alone [ 72 ]. Tamoxifen can also be administered as neoadjuvant treatment, especially for elderly BC patients [ 73 ]. However, studies have demonstrated no difference in OS for ER+ BC patients when neoadjuvant tamoxifen is compared to surgery [ 74 , 75 ].
Other SERMs have since been developed, such as toremifene approved by the FDA in 1997 [ 76 ]. Studies comparing the effect of toremifene and tamoxifen in premenopausal patients with ER+ advanced BC have shown that toremifene efficacy and safety are similar to tamoxifen [ 77 , 78 ]. Bazedoxifene and raloxifene are administered as prevention treatment to postmenopausal patients at high risk of developing invasive BC and for preventing osteoporosis [ 79 , 80 , 81 ].
The most frequent adverse events of SERMs are hot flushes, nausea, vomiting, vaginal bleeding/discharges, and increased risk of thromboembolic events [ 82 ]. Of note, about 40% of HR+ BC patients will develop resistance to SERMs [ 83 ]. SERMs resistance can occur by the loss of ER expression or functions. Epigenetic modifications such as hypermethylation of CpG islands or histone deacetylation can lead to transcriptional repression of ER [ 84 ]. Another potential mechanism for ER expression loss is the overpopulation of ER-negative cells in heterogenous ER+ tumors [ 85 ]. Mutations in the ligand-binding domain of ER gene ( ESR1 ) inhibit the binding of estrogen to the ER leading to the abolition of downstream signaling. Moreover, abnormal splicing can lead to truncated, nonfunctional ER protein [ 86 , 87 ]. Another explanation for SERMs resistance is the abnormal expression of ER coregulators [ 88 ]. Coregulators are very important in the ER pathway as they can increase or decrease ER activity depending on incoming signals [ 89 ]. The most studied coregulator involved in SERMs resistance is the AIB1 (Amplified in breast cancer 1) coactivator protein, often overexpressed in resistant breast tumors [ 90 ]. In particular, in ER+ cells that overexpress HER2, there is a crosstalk between HER2 and AIB1. HER2 induces phosphorylation of AIB1 leading to evasion and subsequent activation of the ER signaling pathway even though it is inhibited by SERMs [ 91 ]
3.1.2. Selective Estrogen Receptor Degraders (SERDs)
To counteract the large proportion of tamoxifen-resistant tumors, a new type of therapeutic agents with a different mechanism of action has been developed: SERDs. In contrast to SERMs, SERDs completely block the ER signaling pathway.
Fulvestrant is the main SERD administered. It was discovered by Wakeling and collaborators in 1987 and demonstrated pure anti-estrogen activity [ 92 ]. Fulvestrant binds to ER with a higher affinity than tamoxifen. Once it binds to the ER, it inhibits receptor dimerization and then blocks ER translocation to the nucleus leading to its degradation [ 93 , 94 , 95 ].
Fulvestrant is administered by intramuscular injections, and common adverse effects are nausea, pain, and headaches [ 96 ]. Fulvestrant is approved to treat postmenopausal and premenopausal patients with ovarian function suppression, with ER+ advanced or metastatic BC on prior endocrine therapy [ 97 ]. More recently (in 2017), fulvestrant was approved as first-line monotherapy for advanced ER+ breast cancer [ 98 ]. According to the 2021 NCCN guidelines, fulvestrant combined with endocrine therapy or CDK4/6 inhibitors is one of the preferred regimens for second-line therapy in ER+ advanced or metastatic BC [ 99 ]. The combination of fulvestrant with other endocrine therapies has not shown any advantages over fulvestrant used in monotherapy [ 100 , 101 ]. Clinical studies have shown benefits from fulvestrant when administered in higher doses to patients with ESR1 -mutated advanced BC [ 102 , 103 ]. Indeed, ESR1 mutations occur in nearly 20% of cases of ER+ BC [ 86 ].
However, fulvestrant can lead to resistance by different mechanisms. For example, by upregulating the PI3K (phosphatidylinositol 3-kinase), mTOR (mammalian target of rapamycin) and Ras-ERK (extracellular signal-regulated kinase) signaling pathways. PI3K/Akt/mTOR is a downstream signaling pathway of ER activation and plays an important role in antiestrogen therapy resistance [ 104 ]. PI3K pathway activation can occur independently of ER by binding to the epidermal growth factor (EGF) [ 105 ]. Moreover, it has been shown that Akt overexpression leads to fulvestrant resistance [ 106 ]. IGF-1R activation (insulin-like growth factor 1 receptor) may be another mechanism of resistance to fulvestrant. IGF-1R expression is involved in cell survival and promotes metastatic cell proliferation. The interaction between IGF-1R and ER initiates the activation of IGF-1R/MAPK (mitogen-activated protein kinase) and IGF-1R/PI3K signaling leading to antiestrogen resistance [ 107 ].
3.1.3. Aromatase Inhibitors (AIs)
Aromatase is a cytochrome P50 enzyme involved in the synthesis of androgens and estrogens [ 108 ]. Aromatase is found in the breast, uterus, and other estrogen-sensitive tissues in specific levels depending on menopausal status [ 109 , 110 ]. Aromatase expression is increased in breast tumors and associated with high estrogen levels. Therefore, high expression of aromatase promotes ER+ tumor proliferation [ 111 ].
Aromatase inhibitors (AIs) block aromatase enzyme activity, leading to the inhibition of estrogen synthesis. Current AIs can be classified into two categories: steroidal AIs and non-steroidal AIs [ 112 ]. Exemestane, a steroidal AI, has a steroid-like structure similar to androstenedione, which is the aromatase substrate. Exemestane irreversibly binds to the aromatase substrate-binding site leading to its inactivation [ 113 ]. Non-steroidal AIs include letrozole and anastrozole. They both bind non-covalently and competitively to the aromatase substrate-binding site and prevent the binding of androgens by saturating the binding site [ 112 ].
AIs are an oral treatment administered only to postmenopausal women (including patients that become postmenopausal following ovarian suppression). It is administered alone or in combination with tamoxifen as adjuvant therapy for HR+ BC patients [ 114 , 115 , 116 , 117 ]. AIs can be administered for 5 years or 2–3 years if followed by tamoxifen and up to 5 years after previous tamoxifen or AI treatment. For advanced or metastatic HR+ BC, AIs can be delivered as first-line and second-line therapy. Patients who become postmenopausal after or during the 5 years of tamoxifen treatment can receive AIs, such as letrozole, as an extended treatment strategy [ 118 , 119 ].
Estrogens have protective effects on the cardiovascular system by regulating serum lipids concentrations and increasing vasodilatation [ 120 ]. Hence, AIs might increase the risk of developing cardiovascular diseases by reducing estrogen levels in the blood [ 121 ]. Other adverse effects of AIs include hot flushes, vaginal dryness, fatigue, and osteoporosis [ 122 ]. ER+ tumors can acquire AI resistance. Some mechanisms of AI resistance are similar to those conferring SERM or SERD resistance, such as ESR1 mutations, epigenetic modifications, and PI3K pathway upregulation [ 123 ]. However, other mechanisms of action are involved in AI resistance. For example, the upregulation of cyclin-dependent kinase 4 (CDK4) or cyclin-dependent kinase 6-retinoblastoma (CDK6-RB) pathways can lead to an estrogen-dependent cell progression [ 124 ]. Clinical studies have shown better benefits from CDK4-CDK6 inhibitors in combination with AIs compared to AIs alone [ 125 , 126 ].
Endocrine therapy is a well-established treatment strategy for HR+ tumors. Over the last decades, SERMs, SERDs and AIs have been proven as safe and effective personalized therapy for HR+ BC patients, and these therapeutic strategies have shown continued improvements. However, the main drawback of endocrine therapy is acquired or de novo resistance [ 127 ]. Hence, it is essential to develop new therapeutic agents that use different modes of action to treat HR+ BC more efficiently.
3.2. Anti-HER2 Therapy
The overexpression of HER2 is associated with worse survival outcome compared to HR-positive/HER2-negative BC [ 128 , 129 ]. Hence, therapies targeting HER2 are essential to treat HER2-positive BC. The current anti-HER2 therapies comprise antibodies that target specific HER2 epitopes, tyrosine kinase inhibitors (TKIs) and, more recently, antibody-drug conjugates (ADCs) [ 130 ]. Anti-HER2 mechanisms of action and resistance are described in Figure 4 .
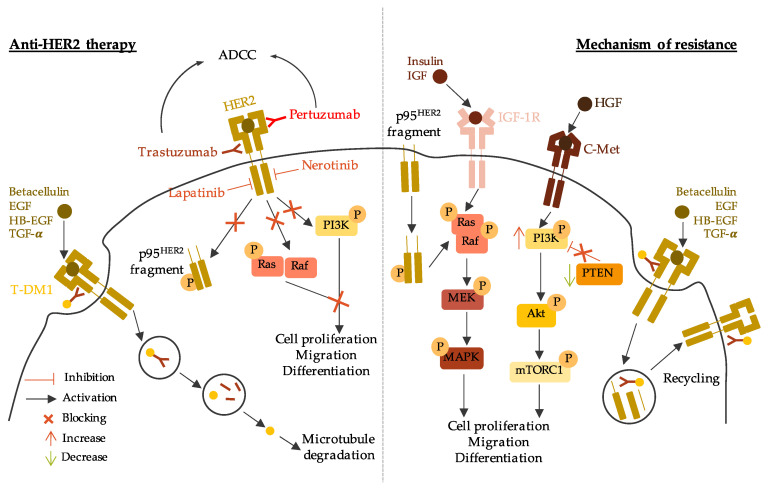
Anti-HER2 therapy mechanisms of action and resistance. The left part of the figure describes the mechanism of action of anti-HER2 therapy through anti-HER2 antibody (trastuzumab and pertuzumab), tyrosine kinase inhibitors (lapatinib and nerotinib), and trastuzumab-emtansine (T-DM1). The right part of the figure describes the mechanism of resistance to anti-HER2 therapy through constitutive active p95 HER2 fragment, activation of other signaling pathways, and rapid recycling of HER2-T-DM1. ADCC: antibody-dependent cellular cytotoxicity; HER2: human epidermal growth factor receptor 2; EGF: epidermal growth factor, HB-EGF: heparin-binding EGF-like growth factor; TGF-α: transforming growth factor alpha; T-DM1: trastuzumab-emtansine; IGF-1R: insulin growth factor receptor 1; IGF: insulin growth factor; HGF: hepatocyte growth factor; MEK/MAPK: mitogen activated protein kinase; PI3K: phosphoinositide 3-kinase; mTOR: mammalian target of rapamycin; PTEN: phosphatase and tensin homolog.
3.2.1. Antibodies Targeting HER2
The first developed HER2-targeted antibody, trastuzumab (Herceptin), was approved by the FDA in 1998 [ 131 , 132 ]. Trastuzumab targets subdomain IV of the HER2 extracellular domain. However, the mechanism underlying trastuzumab’s therapeutic effect is not well understood. Multiple studies have reported hypotheses to explain trastuzumab’s mechanism of action. For instance, trastuzumab may inhibit the formation of the HER2-HER3 heterodimer, known to be the most oncogenic pair in the HER family [ 133 ]. It could also inhibit the formation of the active p95 HER2 fragment by preventing cleavage of the HER2 extracellular domain [ 134 ]. An indirect antitumor effect could be activating antibody-dependent cellular cytotoxicity (ADCC) by engaging with Fc receptors on immune effector cells [ 135 ].
Initially, trastuzumab was approved for administration in metastatic HER2+ BC, increasing the clinical benefits of first-line chemotherapy [ 132 ]. Trastuzumab has also demonstrated its efficacy and safety in early-stage HER2+ BC. It is given as neoadjuvant or adjuvant therapy in combination with other anti-HER2 treatments and/or with chemotherapy [ 136 , 137 , 138 ]. The recommended dose for intravenous trastuzumab is 4 mg/kg followed by 2 mg/kg weekly for 1 year in the adjuvant setting for early-stage HER2+ BC and until disease-free progression for metastatic HER2+ BC [ 139 ].
Pertuzumab (Perjeta) is another antibody that targets the HER2 extracellular domain but binds to subdomain II. Once it binds to HER2, pertuzumab prevents HER2 heterodimerization with other HER family members, leading to inhibition of downstream signaling pathways [ 140 ]. Like trastuzumab, one of pertuzumab’s indirect antitumor effects is activating the ADCC pathway [ 141 ]. Multiple clinical trials have shown that pertuzumab, combined with trastuzumab and chemotherapy, improved OS in metastatic HER2+ BC patients compared to trastuzumab and chemotherapy alone [ 142 , 143 , 144 , 145 ]. The benefits of pertuzumab have also been shown in early-stage HER2+ BC, as pertuzumab can be used in the neoadjuvant or adjuvant setting combined with trastuzumab and chemotherapy [ 146 , 147 , 148 , 149 ]. Pertuzumab is administered in fixed doses of 840 mg followed by 420 mg every three weeks [ 150 ].
Despite the major positive impacts of trastuzumab and pertuzumab in HER2+ BC treatment, only one-third of BC patients with HER2+ tumors benefit from anti-HER2 antibodies [ 151 ]. One of the hypotheses explaining this resistance concerns structural modifications of HER2, which hinder antibody binding. Alternative splicing can lead to a truncated isoform lacking the extracellular domain, thus forming a constitutive active p95 HER2 fragment [ 152 ]. The overexpression of other tyrosine kinases can bypass the signaling pathways mediated by HER2. It has been shown that cells overexpressing IGF-1R overcome cell cycle arrest by increasing CDK2 kinase activity [ 153 ]. Moreover, the overexpression of c-Met (a hepatic growth factor receptor) synergizes with HER2 signaling to confer resistance to anti-HER2 antibodies. Indeed, c-Met physically interacts with HER2, and c-Met depletion renders cells more sensitive to trastuzumab [ 154 , 155 ]. Another hypothesis for anti-HER2 antibody resistance is intracellular alterations in HER2 downstream signaling pathways. HER2 activates PI3K/Akt signaling, and PTEN (phosphatase and tensin homolog) is a well-known inhibitor of this pathway [ 156 ]. Tumors with a loss of PTEN function and/or constitutive activation of PI3K due to alteration mutations achieve worse therapeutic outcomes with trastuzumab [ 157 , 158 ].
3.2.2. Tyrosine Kinase Inhibitors (TKIs)
Since tumors may be resistant to anti-HER2 antibodies, new approaches have been developed. TKIs such as lapatinib, neratinib, or pyrotinib are small molecules that compete with ATP at the catalytic domain of the receptor to prevent tyrosine phosphorylation and HER2 downstream signaling [ 159 ].
Lapatinib is a dual EGFR/HER2 TKI blocking both HER1 and HER2 activation [ 160 ]. In metastatic BC, clinical trials have shown that lapatinib offers more benefits than chemotherapy alone [ 161 , 162 , 163 ]. The effects of lapatinib in the neoadjuvant/adjuvant setting have also been evaluated. As a neoadjuvant treatment, lapatinib plus trastuzumab combined with chemotherapy were more efficient than chemotherapy combined with lapatinib or trastuzumab alone [ 164 ]. Lapatinib as adjuvant treatment showed modest antitumor efficacy compared to placebo in a randomized, controlled, and multicenter phase III trial (TEACH) [ 165 ]. For luminal B (ER/PR+; HER2+) advanced or metastatic BC, lapatinib can be administered in combination with AIs.
Neratinib is an irreversible TKI targeting HER1, HER2, and HER4 [ 166 ]. The FDA approved Neratinib in 2017 as an extended adjuvant treatment for patients with HER2+ early-stage BC and combination with trastuzumab in the adjuvant setting [ 167 , 168 ]. Neratinib can be delivered in combination with capecitabine as a third-line and beyond therapy for HER2+ advanced or metastatic BC.
More recently, pyrotinib, a new generation TKI targeting HER1, HER2 and HER4, has been developed [ 169 ]. Pyrotinib is still under clinical trials to prove its efficacy and safety [ 170 ]. However, in 2018, the Chinese State Drug Administration approved pyrotinib in combination with or after chemotherapy treatment for patients with HER2+ advanced or metastatic BC [ 171 ].
Despite the recent development of TKI treatments, patients can still exhibit intrinsic or acquired resistance to these agents. Three mechanisms of action have been hypothesized: (1) activation of compensatory pathways, (2) HER2 tyrosine kinase domain mutation, and (3) other gene amplification [ 172 ]. For instance, activation of the PI3K/Akt pathway and FOXO3A (Forkhead transcription factor) by the upregulation of HER3 can lead to lapatinib resistance [ 173 ]. Other tyrosine kinases can be involved, such as c-Met, also known to be implicated in trastuzumab resistance. C-Met induces the activation of PI3K/Akt signaling in lapatinib-resistant BC [ 174 ]. Mutations in the HER2 tyrosine kinase domain lead to the constitutive activation of HER2 by substituting individual amino acids [ 175 ]. Lastly, it has been shown that the amplification of the NIBP (TRAPPC9, Trafficking Protein Particle Complex 9) gene occurs in HER2+ lapatinib-resistant tumors. The inhibition of NIBP makes resistant cells sensitive to lapatinib [ 176 ].
3.2.3. Trastuzumab-Emtansine (T-DM1)
Trastuzumab-emtansine (T-DM1) is an antibody-drug conjugate (ADC), which is a conjugate of trastuzumab and a cytotoxic molecule, DM1, a derivative of maytansine [ 177 ]. T-DM1 binds to HER2 with the trastuzumab part. The formed complex is then internalized for degradation, releasing DM1 metabolites into the cytoplasm. DM1 then inhibits microtubule assembly causing cell death [ 178 , 179 ]. Thus, T-DM1 consists of the antitumor effects of trastuzumab and those associated with DM1 metabolites [ 180 ].
Three phase III clinical trials have evaluated the safety and efficacy of T-DM1 for HER2+ metastatic BC [ 181 , 182 , 183 ]. They have shown that T-DM1 improves OS and DFS of HER2+ metastatic BC patients compared to lapatinib in combination with trastuzumab or chemotherapy [ 181 , 182 , 183 ]. T-DM1 as neoadjuvant treatment has less efficacy compared with trastuzumab or pertuzumab with chemotherapy [ 146 ]. This suggests that T-DM1 should not be administered as a neoadjuvant treatment but as a first-line or second-line therapy for HER2+ metastatic BC. The 2021 NCCN guidelines recommend using T-DM1 as second-line therapy for HER2+ advanced or metastatic BC [ 99 ].
The mechanism of action of T-DM1 involves those related to trastuzumab and DM1, so the observed resistance to T-DM1 could come from interference in one or both constituents [ 184 ]. The mechanism of T-DM1 resistance has been hypothesized to involve (1) the loss of trastuzumab mediated activity, (2) the dysfunctional intracellular trafficking of T-DM1, and (3) the impairment of DM1 mediated cytotoxicity [ 185 ].
As previously described in this review, the reduction of trastuzumab effects can occur by reduced HER2 expression, dysregulation of PI3K signaling, or the activation of alternative tyrosine kinase receptors [ 153 , 154 , 156 , 186 ]. The alteration of HER2-T-DM1 complex internalization can go through a rapid recycling of HER2 to the plasma membrane leading to the inhibition of DM1 metabolism released into the cytoplasm [ 187 ]. The internalization of the HER2-T-DM1 complex occurs through the formation of lysosomes. These vesicles enclose lysosomal enzymes involved in HER2-T-DM1 complex degradation. In T-DM1-resistant tumors, the level of lysosomal enzymes is inhibited [ 188 , 189 ]. T-DM1 also disrupts microtubule assembly causing incomplete spindle formation resulting in mitotic catastrophe and apoptosis [ 190 ]. Cells resistant to T-DM1 can avoid this process by reducing the induction of Cyclin-B1, an enzyme essential for cell cycle progression [ 191 ].
HER2+ BC are aggressive and associated with poor prognosis and metastasis, and recurrences. Anti-HER2 therapy has greatly improved the management of HER2+ BC. However, 25% of early-stage HER2+ BC patients will have a recurrence after the initial anti-HER2 treatment [ 192 ]. The emergence of new therapeutic agents specific for HER2+ BC provides new hope to treat this particularly aggressive BC subtype.
3.3. PARP Inhibitors
The prevalence of BRCA (Breast Cancer genes) mutations in TNBC patients is approximately 20% [ 193 ]. BRCA1 and BRCA2 are proteins involved in the DNA damage response to repair DNA lesions [ 194 ]. Mutations in BRCA 1/2 genes are associated with an increased risk of breast and ovarian cancers [ 195 ].
PARP (poly-(ADP-ribose) polymerase protein) proteins are also involved in the DNA damage response as they recruit DNA repair proteins, such as BRCA1 and BRCA2, to the damage site [ 196 ]. PARP inhibitors (PARPi) were developed to inhibit DNA repair in BRCA-mutated BC since cells defective in BRCA functions cannot repair DNA damage when PARP is inhibited [ 197 ]. The principal PARPis currently in clinical development are olaparib, talazoparib, veliparib, and rucaparib [ 198 ]. PARP inhibitors mechanisms of action and resistance are described in Figure 5 .
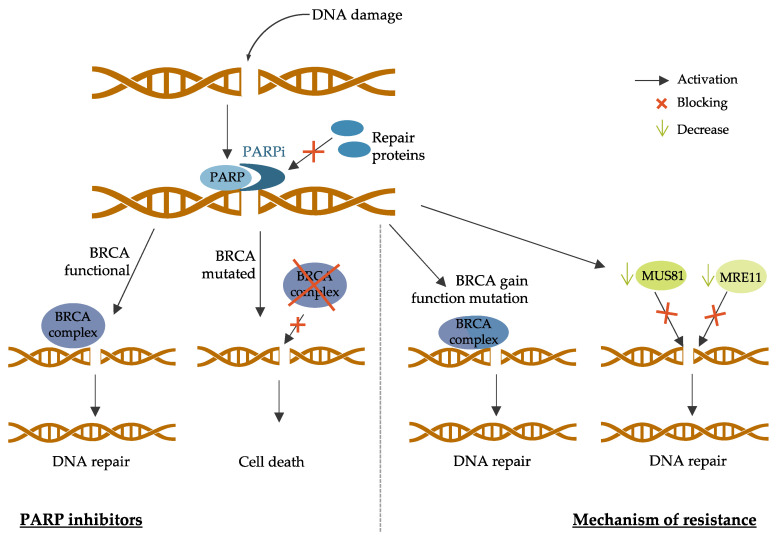
PARP inhibitors mechanisms of action and resistance. The left part of the figure describes the mechanism of PARP inhibitors in the context of BRCA mutated breast cancer. The right part of the figure describes the mechanism of resistance to PARP inhibitors through secondary intragenic mutations restoring BRCA proteins functions and the decrease of the recruitment of nucleases (MUS81 or MRE11) to protect the replication fork. PARP: poly-(ADP-ribose) polymerase protein; PARPi: PARP inhibitors; BRCA: breast cancer protein; MUS81: methyl methanesulfonate ultraviolet sensitive gene clone 81; MRE11: meiotic recombination 11.
3.3.1. Olaparib
Olaparib is the first FDA-approved PARPi for the treatment of BRCA -mutated BC [ 199 ]. Phase I and phase II trials evaluating the effects of olaparib monotherapy in germline BRCA-mutated (gBRCAm) BC proved its clinical benefits by improving progression-free survival (PFS) [ 200 , 201 , 202 , 203 ]. The phase III, randomized, open-label, OlympiAD trial compared olaparib monotherapy vs. standard chemotherapy in patients with BRCA mutated HER2-negative BC. This trial showed that olaparib has better efficacy and tolerability than standard chemotherapy for this group of patients [ 204 ]. Olaparib has also been tested in combination with chemotherapy. A phase I study evaluated the effects of olaparib in combination with paclitaxel in unselected TNBC patients [ 205 ]. The overall response rate (ORR) for these patients was 37%. Two phase I studies evaluating the combination of olaparib with cisplatin or carboplatin in gBRCAm BC patients showed improved ORR [ 206 , 207 ].
3.3.2. Talazoparib
Talazoparib has the highest PARP-DNA trapping efficiency among the PARPis [ 208 ]. A phase II trial testing the effects of talazoparib on gBRCAm early-stage BC showed decreased tumor size in all patients included [ 209 ]. Other phase I and II trials with gBRCAm BC patients receiving talazoparib confirmed the efficiency of this PARPi [ 210 , 211 ]. The EMBRACA study, an open-label phase III trial, compared talazoparib monotherapy to chemotherapy in gBRCAm, HER2-negative BC patients [ 212 ]. PFS and ORR were improved with talazoparib compared to chemotherapy alone.
3.3.3. Veliparib
Veliparib has been mostly evaluated in combination with chemotherapy. For example, the phase II multicenter I-SPY2 trial tested the combination of veliparib and neoadjuvant chemotherapy in unselected TNBC patients [ 213 ]. The predicted complete response rate (pCR) was 51% with veliparib and chemotherapy vs. 26% in the control arm (chemotherapy alone). The phase II BROCADE study evaluated the combination of veliparib with carboplatin and paclitaxel in gBRCAm BC patients [ 214 ]. The ORR was improved with the combination of veliparib and chemotherapy compared to chemotherapy alone. Lastly, the phase III BRIGHTNESS study evaluated the addition of veliparib to carboplatin in the standard neoadjuvant chemotherapy setting [ 211 ]. The addition of veliparib showed no further benefit to chemotherapy.
3.3.4. Rucaparib
Rucaparib is the second PARPi that has been FDA approved for gBRCAm BC patients [ 215 ]. Intravenous rucaparib was tested in a phase II trial of gBRCAm BC patients [ 216 ]. Stable disease, meaning no tumor development, was reported in 44% of patients. Rucaparib was also tested in combination with chemotherapy in unselected TNBC patients [ 217 ]. This phase I study showed that rucaparib could be safely used in combination with chemotherapy. The phase II, a randomized BRE09-146 trial, evaluated rucaparib in combination with cisplatin vs. cisplatin alone in gBRCAm patients with residual disease following neoadjuvant therapy [ 218 ]. DFS was similar in the two arms, as low-dose rucaparib did not affect cisplatin toxicity. However, the rucaparib dose may not have been sufficient to inhibit PARP activity.
Tumor cells can become resistant to PARPi by different mechanisms [ 219 ].
First, secondary intragenic mutations that restore BRCA proteins functions can lead to PARPi resistance [ 220 ]. These genetic events can lead to the expression of nearly full-length proteins or full-length wild-type proteins with complete restored functions [ 221 ]. This has been reported mostly in ovarian cancer patients, and it has also been demonstrated in BC cell line models [ 222 ]. Tumor cells with missense mutations conserving the N-terminal and C-terminal domains of BRCA proteins also lead to poor PARPi response [ 223 ]. Another mechanism of action leading to PARPi resistance is decreased expression of PARP enzymes. Indeed, tumor cells with low PARP1 expression acquire resistance to veliparib [ 224 ].
In addition, tumor cells can find alternative mechanisms to protect the replication fork. It has been shown that PARPi-resistant cells can reduce the recruitment of the MRE11 (meiotic recombination 11) nuclease to the damage site, leading to the protection of the fork by blocking its access [ 225 ]. Another study has shown that BRCA2 -mutated tumors acquired PARPi resistance by reducing the recruitment of the MUS81 (methyl methanesulfonate ultraviolet sensitive gene clone 81) nuclease to protect the replication fork [ 226 ].
Chemotherapy has been the pioneer treatment strategy for TNBC for decades. The development of PARPis has been a major improvement in the treatment of TNBC and, more specifically, gBRCAm TNBC, as they have shown more benefits over chemotherapy [ 227 ]. However, TNBC is a heterogenous BC subtype, and PARPis cannot treat all TNBCs as it is administered only for gBRCAm TNBC [ 228 ]. Therefore, it is necessary to develop specific targeted therapies to treat each TNBC subtype.
4. New Strategies and Challenges for Breast Cancer Treatment
4.1. emerging therapies for hr-positive breast cancer.
As mentioned in Section 3.1 , the major mechanisms of action of current endocrine therapy resistance occur via (1) the mTOR/PI3K/Akt signaling pathway and (2) the actors of the cell cycle progression CDK4/6. Therefore, emerging therapies for HR+ BC mainly target these pathways to bypass estrogen-independent cell survival [ 229 ]. The most recent completed clinical trials on emerging therapies for HR+ BC are presented in Table 1 .
Most recent completed clinical trial on emerging therapies for HR-positive breast cancer.
HR+: hormone receptors positive; HER2-: human epidermal growth factor receptor 2 negative; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; pCR: pathologic complete response; HR: hazard ratio.
4.1.1. mTOR/PI3K/AKT Pathway Inhibitors
The mTOR/PI3K/Akt pathway inhibitors can be divided into different categories according to the target in the pathway. Specific inhibitors have been developed to target all or specific isoforms of PI3K, mTORC1 and Akt [ 251 ].
Pan-Pi3K Inhibitors
Pan-PI3K inhibitors target all PI3K isoforms resulting in significant off-target effects. The main pan-PI3K inhibitors are buparlisib and pictilisib [ 252 ]. Multiple clinical trials have tested the effects of pan-PI3K inhibitors in luminal BC.
The phase III randomized double-blinded BELLE-2 trial compared buparlisib combined with fulvestrant, to fulvestrant monotherapy in luminal A advanced or metastatic BC patients [ 230 ]. The results of this trial showed a modest improvement in PFS when buparlisib was added to fulvestrant. Another phase III clinical trial (BELLE-3) studied the effects of buparlisib plus fulvestrant in luminal A advanced or metastatic BC patients with no benefits from endocrine therapy [ 231 ]. Though PFS was significantly improved with buparlisib, there were severe adverse effects such as hyperglycemia, dyspnea, or pleural effusion. Lastly, the phase II/III BELLE-4 clinical trial evaluated buparlisib plus paclitaxel in HER2-negative locally advanced or metastatic BC patients [ 232 ]. The addition of buparlisib to paclitaxel did not improve PFS in these patients. Thus, further studies on buparlisib in HR+ BC were not conducted. The phase II randomized, double-blinded FERGI clinical trial analyzed the effects of pictilisib plus fulvestrant in luminal A BC patients resistant to AI [ 233 ]. The addition of pictilisib to fulvestrant did not improve PFS. Moreover, severe adverse effects occurred when the dose of pictilisib was increased. These results were confirmed for pictilisib plus paclitaxel, as the phase II PEGGY study showed no benefit from pictilisib in PI3K-mutated HER2-negative BC patients [ 234 ].
Hence, pan-PI3K inhibitors are not optimal to treat HR+ BC due to their toxicity and lack of efficacy.
Isoform-Specific PI3K Inhibitors
To sort out issues related to off-target effects and toxicities with pan-PI3K inhibitors, isoform-specific PI3K inhibitors have been developed. These isoform-specific PI3K inhibitors can specifically target the PI3K p110α, p110β, p110δ, and p110γ isoforms [ 252 ]. Multiple clinical trials have tested the effects of isoform-specific PI3K inhibitors.
PI3K p110α is the most commonly mutated isoform in BC [ 253 ]. Alpelisib is the first FDA-approved PI3K p110α isoform inhibitor. A phase Ib clinical trial tested the effects of alpelisib and letrozole in patients with ER+ metastatic BC refractory to endocrine therapy [ 235 ]. The clinical benefit of the alpselisib and letrozole combination was higher for patients with PI3K-mutated BC, but clinical activity was still observed in patients with non-mutated tumors. The phase III randomized SOLAR-1 clinical trial compared the effects of alpelisib plus fulvestrant to fulvestrant alone in luminal A advanced BC patients who received no benefits from prior endocrine therapy [ 236 ]. The addition of alpelisib improved PFS for patients with PI3K-mutated BC.
Taselisib targets the PI3K p110α, p110γ and p110δ isoforms [ 254 ]. Taselisib was tested in the SANDPIPER study, a phase III randomized clinical trial, in combination with fulvestrant in patients with ER+ metastatic BC resistant to AIs [ 238 ]. Although the addition of taselisib slightly improved PFS, further clinical trials with taselisib were interrupted since high rates of severe adverse events were detected.
mTORC1 Inhibitors
mTORC1 inhibitors, such as everolimus, block the mTORC1 dependent phosphorylation of s6k1 [ 255 ]. The BOLERO-2 phase III randomized clinical trial investigated the effects of exemestane with or without everolimus in AI-resistant ER+ metastatic BC patients [ 240 ]. The combination of everolimus and exemestane improved PFS. The TAMRAD phase II randomized open-label study compared the effects of tamoxifen with or without everolimus in AI-resistant luminal A BC patients [ 241 ]. This study showed an improvement in overall survival (OS) when everolimus was given in combination with tamoxifen. The findings of these two clinical trials led to FDA approval of everolimus. More recently, the PrE0102 phase II randomized clinical trial showed that the addition of everolimus to fulvestrant improved PFS of patients with AI-resistant ER+ BC compared to fulvestrant alone [ 242 ].
Akt Inhibitors
Akt inhibitors target all Akt isoforms as Akt 1, 2, and 3 isoforms share very similar structures [ 256 ]. Capivasertib is the principal Akt inhibitor under investigation in different clinical trials. The FAKTION phase II multi-centered randomized clinical trial compared the effects of capivasertib plus fulvestrant to fulvestrant plus placebo in AI-resistant luminal A advanced BC patients [ 243 ]. PFS was significantly improved with the combination of capivasertib and fulvestrant in comparison with the placebo arm.
The AKT1 E17K activating mutation is the most common in Akt and occurs in approximately 7% of ER+ metastatic BC. This mutation in the Akt lipid-binding pocket leads to constitutive Akt activation by modifying its localization to the membrane [ 257 ]. A phase I study analyzed the effects of capivasertib alone or in combination with fulvestrant in a cohort of patients with AKT1 E17K mutation ER+ metastatic BC [ 244 ]. Capivasertib, in combination with fulvestrant, demonstrated clinically meaningful activity and better tolerability compared to capivasertib alone.

4.1.2. CDK4/6 Inhibitors
There are currently three CDK4/6 inhibitors approved to treat HR+/HER2- metastatic BC: palbociclib, ribociclib, and abemaciclib. They can be administered as first-line treatment combined with AIs or as second-line treatment combined with fulvestrant [ 258 ].
First-Line Treatment
Palbociclib, a highly selective CDK4/6 inhibitor, is the first FDA-approved CDK4/6 inhibitor as first-line treatment combined with AIs for metastatic or advanced HR+ BC patients [ 259 ].
PALOMA-1 is an open-label, randomized phase II study that evaluated the effects of palbociclib in combination with letrozole vs. letrozole alone as first-line treatment for HR+ advanced BC patients [ 126 ]. The addition of palbociclib to letrozole significantly improved PFS in HR+ BC patients. A phase III study was performed (PALOMA-2) to confirm these findings and expand the efficacy and safety of palbociclib, [ 245 ]. This double-blinded clinical trial tested the combination of palbociclib and letrozole in postmenopausal BC patients without prior systemic therapy for advanced BC. The addition of palbociclib to letrozole significantly improved PFS and ORR.
Ribociclib is the second FDA-approved CDK4/6 inhibitor for first-line treatment in postmenopausal advanced BC patients in combination with AIs [ 260 ]. The phase III MONALEESA-2 clinical trial results showed improved PFS and ORR with the combination of ribociclib and letrozole in HR+ metastatic BC patients. The clinical benefits and manageable tolerability observed with ribociclib and letrozole are maintained with longer follow-up compared to letrozole alone [ 247 ].
Abemaciclib has been tested in the phase III randomized double-blinded MONARCH-3 study [ 250 ]. HR+ advanced BC patients with no prior systemic therapy received abemaciclib plus anastrozole or letrozole or AIs plus placebo in the control arm. PFS and ORR were significantly improved with the combination of abemaciclib and AIs.
Second-Line Treatment
As second-line treatment, palbociclib can be given in combination with fulvestrant in advanced or metastatic BC patients with disease progression after endocrine therapy [ 261 ]. This was confirmed in the phase III multi-centered randomized double-blinded PALOMA-3 trial [ 246 ]. BC patients who received palbociclib plus fulvestrant had significantly longer PFS compared to fulvestrant plus placebo.
The phase III MONALEESA-3 study tested the effects of ribociclib plus fulvestrant in patients with HR+ advanced BC who received prior endocrine therapy in the advanced setting [ 248 ]. The PFS and ORR were significantly improved when ribociclib was added to fulvestrant. Thus, ribociclib plus fulvestrant can be considered as second-line treatment for these BC patients.
Abemaciclib has been recently approved in combination with fulvestrant for HR+ advanced or metastatic BC patients with disease progression after endocrine therapy. This was based on the results of the phase III, double-blinded MONARCH 2 study [ 249 ]. The combination of abemaciclib and fulvestrant demonstrated a significant improvement of PFS and ORR compared to fulvestrant plus placebo in HR+ metastatic BC patients who experienced relapse or progression after prior endocrine therapy.
mTOR/PI3K/Akt inhibitors and CDK4/6 inhibitors show great promise for advanced HR+ BC resistant to endocrine therapy. To leverage the potential of these two types of therapies, some preclinical studies have evaluated a triple therapy combination including PI3K inhibitors, CDK4/6 inhibitors, and endocrine therapy (see the summarized table at the end of the manuscript) [ 262 ].
4.2. New Strategic Therapies for HER2-Positive Breast Cancer
As mentioned in Section 3.2 , HER2+ BC is currently treated with specific HER2 targeting antibodies or tyrosine kinase inhibitors (TKIs), and more recently, with TDM-1, an antibody-drug conjugate. These treatments have greatly improved HER2+ BC survival. However, 25% of HER2+ BC patients will still develop resistance to anti-HER2 treatment. Hence, new therapeutic strategies are emerging, such as new antibodies targeting HER2, new TKIs, vaccines, and PI3K/mTOR and CDK4/6 inhibitors [ 263 ]. The most recent completed clinical trials on new strategies for HER2+ BC treatment are gathered in Table 2 .
Most recent completed clinical trials on emerging therapies for HER2+ breast cancer.
HER2+: human epidermal growth factor receptor 2 positive; ER+: estrogen receptor positive; HLA2/3: human leucocyte antigen 2/3; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; DFS: disease-free survival OS: overall survival GM-CSF: granulocyte macrophage colony-stimulated factor; HR: hazard ratio.
4.2.1. New Antibodies
Novel types of antibodies have been developed to target HER2+ BC more efficiently. They can be divided into three categories: antibody-drug conjugates (ADC), modified antibodies, and bispecific antibodies.
Antibody-Drug Conjugates (ADC)
ADCs are the combination of a specific monoclonal antibody and a cytotoxic drug that is released in the antigen-expressing cells [ 280 ]. The most common ADC is TDM-1, and the promising results with TDM-1 have led to the development of new ADCs.
Trastuzumab-deruxtecan (DS-8201a) is a HER2-targeting antibody (trastuzumab) linked to a DNA topoisomerase I inhibitor (deruxtecan) [ 281 ]. A phase I study demonstrated that DS-8201a had antitumor activity even with HER2 low-expressing tumors [ 282 ]. These results led to phase II and phase III clinical trials. The DESTINY-Breast01 clinical trial is an open-labeled, single-group, multicentered phase II study [ 264 ] was evaluated in HER2+ metastatic BC patients who received prior TDM-1 treatment. DS-8201a showed durable antitumor activity for these patients. Two phase III clinical trials are currently evaluating DS-8201a. DESTINY-Breast02 (ClinicalTrials.gov identifier: NCT03523585 ) is comparing DS-8201a to standard treatment (lapatinib or trastuzumab) in HER2+ metastatic BC patients previously treated with TDM-1. The DESTINY-Breast03 (ClinicalTrials.gov identifier: NCT03529110 ) trial is evaluating the effects of DS-8201a vs. TDM-1 in HER2+ metastatic BC patients with prior trastuzumab and taxane treatment.
Trastuzumab-duocarmycin (SYD985) is a HER-2 targeting antibody (trastuzumab) conjugate with a cleavable linker-duocarmycin payload that causes irreversible alkylation of the DNA in tumor cells leading to cell death [ 283 ]. A dose-escalation phase I study evaluated the effects of SYD85 in BC patients with variable HER2 status and refractory to standard cancer treatment [ 284 ]. Trastuzumab-duocarmycin showed clinical activity in heavily pretreated HER2+ metastatic BC patients, including TDM-1 resistant and HER2-low BC patients. After these promising results, a phase I expansion cohort study was performed on the same type of patients (heavily pretreated HER2+ or HER2-low BC patients) [ 265 ]. This study confirmed previous results on the efficacy of STD985. A phase III clinical trial (TULIP-ClinicalTrials.gov identifier: NCT03262935 ) is ongoing to compare SYD985 to the treatment chosen by the physician in HER2+ metastatic BC patients. Other ADCs are under clinical trials to test their safety and activity for HER2+ advanced BC patients. RC48 is an anti-HER2 antibody conjugated with monomethyl auristatin E that demonstrated promising efficacy and a manageable safety profile in an open-labeled, multicentered phase II study (ClinicalTrials.gov identifier: NCT02881138 ) [ 248 ]. PF06804103 conjugates an anti-HER2 monoclonal antibody and the cytotoxic agent, Aur0101. In a phase I study (ClinicalTrials.gov identifier: NCT03284723 ), PF06804103 showed manageable toxicity and promising antitumor activity [ 249 ]. XMT1522 showed encouraging results in a dose-escalation phase I study (ClinicalTrials.gov identifier: NCT02952729 ) [ 250 ]. MEDI4276, which targets two different HER2 epitopes and is linked to a microtubule inhibitor, showed promising clinical activity in a phase I study (ClinicalTrials.gov identifier: NCT02576548 ) [ 254 ] (see the summarized table at the end of the manuscript).
Chimeric Antibody
Margetuxumab (MGAH22) is a human/mouse chimeric IgG1 targeting HER2 monoclonal antibody. It is based on trastuzumab as it binds to the same epitope (subdomain IV or HER2 extracellular domain) but with an enhanced Fcγ domain. The substitution of five amino acids into the IgG1 Fc domain increases CD16A affinity, a receptor found on macrophages and natural-killer cells, and decreases CD32B affinity, leading to increased antibody-dependent cell-mediated cytotoxicity (ADCC) [ 285 ]. A phase I study evaluated margetuximab toxicity and tumor activity on HER2+ BC patients for whom no standard treatment was available [ 266 ]. This study showed promising single-agent activity of margetuximab as well as good tolerability. The phase III randomized open-labeled SOPHIA clinical trial (ClinicalTrials.gov Identifier: NCT02492711 ) compared margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in pretreated HER2+ advanced BC patients [ 286 ]. The combination of margetuximab and chemotherapy significantly improved the PFS of patients compared to trastuzumab plus chemotherapy. This study is still under investigation to collect data on OS (see the summarized table at the end of the manuscript).
Bispecific Antibodies
Bispecific antibodies (BsAbs) can target two different epitopes in the same or different receptors by combining the functionality of two monoclonal antibodies [ 287 ]. MCLA-128 targets both HER2 and HER3 and have an enhanced ADCC activity [ 288 ]. A phase I/II study evaluated the safety, tolerability, and antitumor activity of MCLA-128 in patients with pretreated HER2+ metastatic BC.
Preliminary results showed encouraging clinical benefits of MCLA-128. An open-labeled, multicentered phase II study (ClinicalTrials.gov identifier: NCT03321981 ) is ongoing to evaluate the effects of MCLA-128 in combination with trastuzumab and chemotherapy in HER2+ metastatic BC patients.
ZW25 is a BsAb biparatopic that binds the IV and II subdomains of the HER2 extracellular domain, the binding epitopes of trastuzumab and pertuzumab, respectively [ 289 ]. The efficacy of ZW25 was evaluated in a phase I study given alone or in combination with chemotherapy in patients with advanced or metastatic HER2+ BC. The results of this study showed promising antitumor activity, and no-dose limiting was observed.
T-cell bispecific antibodies (TCBs) are another type of BsAbs recently developed. TCBs target the CD3-chain of the T-cell receptor and tumor-specific antigens, resulting in lymphocyte activation and tumor cell death [ 290 ].
GBR1302 targets both HER2 and CD3 receptors and directs T-cells to HER2+ tumor cells. A phase II study (ClinicalTrials.gov identifier: NCT03983395 ) is ongoing to determine the safety profile of the GBR1302 single agent in previously treated HER2+ metastatic BC. PRS-343 targets HER2 and the immune receptor CD137, a member of the tumor necrosis factor receptor family. Two clinical trials are investigating the effects of PRS-343 monotherapy (ClinicalTrials.gov identifier: NCT03330561 ) or in combination with other treatments (ClinicalTrials.gov identifier: NCT03650348 ) (see the summarized table at the end of the manuscript).
4.2.2. HER2-Derived Peptide Vaccines
One of the strategies of immunotherapy is activating the patient’s immune system to kill cancer cells. Vaccination is an emerging approach to induce a tumor-specific immune response by targeting tumor-associated antigens, such as HER2 [ 291 ]. HER2-derived peptide vaccines comprise different parts of the HER2 molecule, such as E75 (extracellular domain), GP2 (transmembrane domain), and AE37 (intracellular domain) [ 292 ].
E75 (HER2/neu 369–377: KIFGSLAFL) has high affinity for HLA2 and HLA3 (human leucocyte antigen) that can stimulate T-cells against HER2 overexpressing tumor cells [ 293 ]. The efficacy of the E75 vaccine to prevent BC recurrence has been evaluated in a phase I/II study, in which high-risk HER2+ HLA2/3+ BC patients received the E75 vaccine [ 269 ]. The results demonstrated the safety and clinical efficacy of the vaccine as PFS was improved in the E75-vaccinated group compared to the unvaccinated group. Other clinical trials are currently investigating the efficacy of the E75 vaccine on HER2+ BC (see he summarized table at the end of the manuscript).
GP2 (654-662: IISAVVGIL) is a subdominant epitope with poor affinity for HLA2 [ 294 ]. A phase I trial evaluating the effects of a GP2 vaccine in disease-free BC patients showed that it was safe and tolerable with HER2-specific immune response [ 295 ]. The GP2 vaccine has been tested in a randomized, open-labeled phase II study to prevent BC recurrence. The patients that received the GP2 vaccine had HER2+ and HLA2+ BC and were disease-free with a high risk of recurrence at the time of the study [ 270 ]. The results demonstrated that the GP2 vaccine was safe and clinically beneficial for patients with HER2+ BC who received the full vaccine series.
AE37 (Ii-key hybrid of MHC II peptide AE36 (HER2/neu 776–790)) can stimulate CD8+ and CD4+ cells. A randomized, single-blinded phase II study evaluated the effects of an AE37 vaccine to prevent BC recurrence. Patients with a high risk of recurrence and HER2+ BC received the AE37 vaccine [ 271 ]. The vaccination demonstrated no benefit in the overall intention-to-treat analysis, a method that considers the randomized treatment to avoid bias happening after the randomization [ 296 ]. However, the study showed that the AE37 vaccine was safe, and results suggested that it could be effective for HER2-low BC, such as TNBC.
4.2.3. New Tyrosine Kinase Inhibitors (TKIs)
As previously described in this review (see Section 3.2.2 Tyrosine kinase inhibitors (TKIs)), TKIs are small molecules targeting the HER2 intracellular catalytic domain [ 159 ]. New TKIs have been developed with better efficacy and less toxicity in the treatment of HER2+ metastatic BC, such as tucatinib and poziotinib.
Tucatinib is a TKI with high selectivity for HER2, leading to less EGFR-related toxicities, common with other HER TKIs [ 297 ]. A phase I dose-escalation trial evaluated the combination of tucatinib and trastuzumab in BC patients with progressive HER2+ brain metastases [ 298 ]. This study showed preliminary evidence of tucatinib efficacy and tolerability in these patients. Tucatinib was also tested in combination with TDM-1 in a phase Ib trial in HER2+ metastatic BC patients with heavy pre-treatment [ 299 ]. The combination of tucatinib and TDM-1 showed acceptable toxicity and antitumor activity in these patients. Tucatinib was FDA approved in combination with trastuzumab and capecitabine for patients with advanced or metastatic HER2+ BC who received prior anti-HER2 in the metastatic setting [ 300 ]. This was based on the results of the phase II HER2CLIMB clinical trial, where HER2+ metastatic BC patients received tucatinib or placebo in combination with trastuzumab and capecitabine [ 267 ]. The addition of tucatinib to trastuzumab and capecitabine improved PFS and OS of heavily pretreated HER2+ metastatic BC patients.
Poziotinib is a pan-HER kinase inhibitor that irreversibly inhibits the HER family members’ kinase activity [ 301 ]. A phase I study evaluated the efficacy and tolerability of poziotinib in advanced solid tumors. The results showed encouraging antitumor activity against different types of HER2+ cancers as poziotinib was safe and well-tolerated by the patients [ 302 ]. The phase II NOV120101-203 study evaluated the safety and efficacy of poziotinib monotherapy in heavily pretreated HER2+ metastatic BC patients [ 268 ]. Poziotinib showed meaningful activity in these patients with no severe toxicities.
4.2.4. mTOR/PI3K Inhibitors and CDK4/6 Inhibitors
As mentioned in the previous Section 4.1 , mTOR/PI3K inhibitors and CDK4/6 inhibitors have been evaluated as potential new strategic therapies for HR+ BC resistant to endocrine therapy. The mTOR/PI3K signaling pathway and CDK4/6 also play a role in the mechanisms leading to treatment resistance in HER2+ BC [ 303 ]. Thus, targeting them with mTOR/PI3K and CDK4/6 inhibitors is also being investigated in HER2+ BC subtype.
mTOR/PI3K Inhibitors
Alpelisib and taselisib are PI3K isoform-specific inhibitors that were also evaluated in HR+ BC [ 235 , 236 , 238 , 253 , 254 ]. A phase I study evaluated alpelisib in combination with trastuzumab and LJM716 (a HER3-targeted antibody) in patients with PI3KCA mutant HER2+ metastatic BC [ 272 ]. Unfortunately, the results of this study were limited by high gastrointestinal toxicity. Another phase I study tested alpelisib in combination with TDM-1 in HER2+ metastatic BC patients pretreated with trastuzumab [ 273 ]. The combination of alpelisib and TDM-1 demonstrated tolerability and antitumor activity in trastuzumab-resistant HER2+ metastatic BC patients. Taselisib is being tested in an ongoing phase Ib dose-escalation trial in combination with anti-HER2 therapies (trastuzumab, pertuzumab and TDM-1) in HER2+ advanced BC patients (ClinicalTrials.gov identifier: NCT02390427 ).
Copanlisib is a highly selective and potent pan-class I PI3K inhibitor [ 304 ]. A phase Ib (PantHER) study evaluated the tolerability and activity of copanlisib in combination with trastuzumab in heavily pretreated HER2+ metastatic BC patients [ 274 ]. The combination of copanlisib and trastuzumab was safe and tolerable. Preliminary evidence of tumor stability was observed in these patients.
Everolimus is a mTORC1 inhibitor also tested in HR+ BC [ 240 , 241 , 242 ]. Everolimus was tested in phase III clinical trials, in combination with trastuzumab and docetaxel (BOLERO-1), or in combination with trastuzumab and vinorelbine (BOLERO-3) in trastuzumab-resistant advanced HER2+ BC [ 275 , 276 ]. Unfortunately, results showed an increase of adverse effects with everolimus. Moreover, the BOLERO-1 clinical trial showed no improvement in PFS with the combination of trastuzumab and everolimus. By contrast, PFS was significantly longer when everolimus was added to vinorelbine in BOLERO-3. A study analyzing the molecular alterations found in patients in the BOLERO-1 and BOLERO-3 clinical trials demonstrated that HER2+ BC patients could derive more benefit from everolimus if the tumors had PI3KCA mutations, PTEN loss or a hyperactive PI3K pathway [ 305 ].
CDK4/6 Inhibitors
Palbociclib, ribociclib and abemaciclib are CDK4/6 inhibitors that have been FDA approved to treat HR+ BC as first-line treatments [ 247 , 250 , 259 ]. They have also been evaluated in multiple clinical trials for advanced HER2+ BC. Palbociclib has been tested in combination with trastuzumab in the phase II SOLTI-1303 PATRICIA clinical trial in heavily pretreated advanced HER2+ BC patients [ 277 ]. Palbociclib combined with trastuzumab demonstrated safety and encouraging survival outcomes in these patients. Palbociclib has also been evaluated in combination with TDM-1 in HER2+ advanced BC patients pretreated with trastuzumab and taxane therapy [ 306 ]. The results of this phase I/Ib study showed safety, tolerability, and antitumor activity in these patients.
Ribociclib was evaluated in a phase Ib/II trial in combination with trastuzumab to treat advanced HER2+ BC patients previously treated with multiple anti-HER2 therapies [ 278 ]. The combination of ribociclib and trastuzumab was safe, but there was limited activity in heavily pretreated patients. The conclusions of this study suggest that CDK4/6 inhibitor/anti-HER2 combination should be administered in patients with few previous therapies.
Abemaciclib has been tested in the phase II randomized open-labeled MonarcHER trial in combination with trastuzumab with or without fulvestrant vs. trastuzumab with standard chemotherapy in HR+/HER2+ BC patients [ 279 ]. The combination of abemaciclib, trastuzumab, and fulvestrant significantly improved PFS in these patients, with a tolerable safety profile.
There are multiple ongoing clinical trials for advanced HER2+ BC testing the combination of palbociclib, trastuzumab, pertuzumab, and anastrozole (ClinicalTrials.gov identifier: NCT03304080 ); or palbociclib and trastuzumab plus letrozole (ClinicalTrials.gov identifier: NCT03054363 ). Preliminary results are expected around July 2021 and March 2022, respectively (see he summarized table at the end of the manuscript).
A great proportion of HER2+ BC patients develop resistance to traditional anti-HER2 therapies, and 40–50% of patients with advanced HER2+ BC develop brain metastases [ 307 ]. Thus, developing new therapies to overcome resistance is essential. The therapeutic strategies that have been described in this section provide new hope for HER2+ BC patients, especially for advanced or metastatic HER2+ BC patients.
4.3. Emerging Therapies for Triple Negative Breast Cancer (TNBC)
TNBC is the most aggressive BC subtype. The fact that TNBC lacks ER and PR expression and does not overexpress HER2, combined with its high heterogeneity, has contributed to the difficulties in developing efficient therapies [ 308 ]. Thus, multiple strategic therapies have been developed to treat all TNBC subtypes. These include conjugated antibodies, targeted therapy, and immunotherapy. An overview of the most recent and completed clinical trials on emerging therapies for TNBC is presented in Table 3 .
Most recent completed clinical trials on emerging therapies for TNBC.
TNBC: triple negative breast cancer; HER2: human epidermal growth factor receptor; HR: hormonal receptor; MBC: metastatic breast cancer; BC: breast cancer; AR: androgen receptor; PPV: personalized peptide vaccine; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; IDFS: invasive disease-free survival; OS: overall survival; TTP: time to progression; pCR: pathologic complete response; HR: hazard ratio.
4.3.1. Antibodies-Drug Conjugates (ADC)
Antibody drug conjugates (ADCs) deliver a cytotoxic drug into the tumor cell by the specific binding of an antibody to a surface molecule [ 280 ]. Multiple ADCs have been investigated in TNBC such as sacituzumab govitecan, ladiratuzumab vedotin, or trastuzumab deruxtecan.
Sacituzumab govitecan combines an antibody targeting trophoblast antigen 2 (Trop-2) and a topoisomerase I inhibitor SN-38 [ 334 ]. Trop-2, a CA 2+ signal transducer, is expressed in 90% of TNBCs and is associated with poor prognosis [ 335 , 336 ]. A single-arm, multicentered phase I/II study evaluated sacituzumab govitecan in heavily pretreated metastatic TNBC patients [ 336 , 337 ]. The efficacy and safety of scituzumab govitecan was shown in these patients, as it was associated with durable objective response. Based on these results, a randomized phase III trial (ASCENT) tested sacituzumab govitecan compared to single-agent chemotherapy chosen by the physician in patients with relapsed or refractory metastatic TNBC [ 309 ]. Sacituzumab govitecan significantly improved PFS and OS of metastatic TNBC patients compared to chemotherapy.
Ladiratuzumab vedotin is composed of a monoclonal antibody targeting the zinc transporter LIV-1 and a potent microtubule disrupting agent, monoethyl auristatin E (MMAE) [ 338 ]. LIV-1 is a transmembrane protein with potent zinc transporter and metalloproteinase activity, expressed in more than 70% of metastatic TNBC tumors [ 339 ]. All clinical trials investigating ladiratuzumab vedotin are still ongoing. A dose-escalation phase I study is evaluating the safety and efficacy of ladiratuzumab vedotin in heavily pretreated metastatic TNBC patients (ClinicalTrials.gov identifier: NCT01969643 ). Preliminary results showed encouraging antitumor activity and tolerability of ladiratuzumab vedotin with an objective response rate of 32% [ 340 ]. The estimated study completion date is June 2023. Two phase Ib/II trials are testing ladiratuzumab vedotin in combination with immunotherapy agents in metastatic TNBC patients, such as pembrolizumab (ClinicalTrials.gov Identifier: NCT03310957 ) with expected preliminary results in February 2022, or in combination with multiple immunotherapy-based treatments (ClinicalTrials.gov Identifier: NCT03424005 ) with expected preliminary results in January 2023.
Trastuzumab deruxtecan is an ADC developed as a treatment for metastatic HER2+ BC patients. Its mechanism of action is described in Section 3.2 . Even though trastuzumab deruxtecan was developed to treat HER2+ BC, it showed antitumor activity in HER2-low tumors in a phase I study [ 282 ]. Based on these results, an ongoing open-labeled, multicentered phase III study (ClinicalTrials.gov Identifier: NCT03734029 ) is recruiting patients with HER2-low metastatic BC to test trastuzumab deruxtecan vs. standard treatment chosen by the physician. Preliminary results are expected in January 2023 (see Table 4 ).
Ongoing clinical trials on emerging therapies for BC treatment for all BC molecular subtypes.
TNBC: triple negative breast cancer; HER2: human epidermal growth factor receptor 2; ER: estrogen receptor; MBC: metastatic breast cancer; BC: breast cancer; HR: hormonal receptor; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; DFS: disease-free survival; OS: overall survival; TTP: time to progression. pCR: pathologic complete response; GM-CSF: granulocyte macrophage colony-stimulated factor; DLT: dose-limiting toxicities; MTD: maximum tolerated dose; TTF: time to treatment failure; TTR: time to treatment response; iDFS: invasive disease-free survival; RFS: recurrence free survival; DDFS: distant disease-free survival; iEFS: invasive events-free survival; CR: clinical response; DoCB: duration of clinical benefit; SD: stable disease; DoR: duration of response; IAEs: incidence of adverse events; TDR: treatment discontinuation rate; PR: partial response; DCR: disease control rate; HR: hazard ratio.
4.3.2. Targeted Therapies
Targeted therapy is the current standard of care to treat HR+ and HER2+ BC, but it cannot be administered to patients with TNBC as these tumors lack the expression of these biomarkers. Hence, the next logical step is to identify biomarkers associated with TNBC to develop specific targeted therapies. Several emerging targeted therapies are being clinically trialed with limited or mixed results.
VEGF and EGFR Inhibitors
Vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) are overexpressed in most TNBC patients [ 341 , 342 ]. Bevacizumab and cetuximab are antibodies developed to specifically target VEGF and EGFR, respectively. Unfortunately, clinical trials studying the effects of these antibodies in TNBC patients demonstrated limited results. The phase III, randomized BEATRICE study evaluating adjuvant bevacizumab-continuing therapy in TNBC demonstrated no significant benefit in OS [ 310 ]. A phase II trial evaluating the impact of adding bevacizumab or cisplatin to neoadjuvant chemotherapy to stage II to III TNBC concluded that further investigation of bevacizumab in this setting was unlikely [ 311 ].
The phase II randomized TBCRC 001 trial testing the combination of cetuximab and carboplatin in stage IV TNBC showed a response in fewer than 20% of patients [ 312 ]. Another randomized phase II study compared the effects of cetuximab plus cisplatin to cisplatin alone in metastatic TNBC patients. Adding cetuximab to cisplatin prolonged PFS and OS, warranting further investigation of cetuximab in TNBC [ 313 ]. Based on these results, bevacizumab is not recommended for the treatment of TNBC.
mTOR/PI3K/AKT Inhibitors
mTOR/PI3K/Akt signaling pathway is an important target involving all BC subtypes. Inhibitors of mTOR, PI3K, and Akt have been tested in HR+ and HER2+ BC patients and have also been tested in TNBC patients. The mTOR inhibitor everolimus has been tested in a randomized phase II trial in combination with chemotherapy vs. chemotherapy alone in stage II/III TNBC patients [ 314 ]. Unfortunately, the addition of everolimus was associated with more adverse effects, without improving pCR or clinical response. A phase I study testing the combination of everolimus and eribulin in metastatic TNBC patients showed that this combination was safe, but the efficacy was modest [ 343 ].
The Akt inhibitor ipatasertib has been tested in combination with paclitaxel (vs. placebo) for metastatic TNBC patients in the phase II multicentered double-blinded randomized LOTUS trial [ 315 ]. The results showed improved PFS when patients received ipatasertib. Another phase II double-blinded randomized trial, FAIRLANE, testing neoadjuvant ipatasertib plus paclitaxel for early TNBC, showed no clinically or statistically significant improvement in the pCR rate, but ipatasertib’s antitumor effect was more pronounced in patients with PI3K/AKT1/PTEN-altered tumors [ 316 ]. Capivasertib, another Akt inhibitor, has been tested in combination with paclitaxel (vs. placebo), first-line therapy for metastatic TNBC patients in the phase II double-blinded randomized PAKT trial [ 317 ]. The addition of capivasertib to paclitaxel significantly improved PFS and OS, with better benefits for patients with PI3K/AKT1/PTEN-altered tumors.
Androgen Receptor Inhibitors
The androgen receptor (AR) is a steroidal hormonal receptor that belongs to the nuclear receptor family and is expressed in 10% to 50% of TNBC tumors [ 344 ]. Tumors expressing AR have better prognosis but are less responsive to chemotherapy [ 345 ]. Multiple clinical trials have tested AR inhibitors in TNBC [ 318 , 319 , 320 ].
Bicalutamide, an AR agonist, was tested in a phase II study in patients with AR+, HR- metastatic BC [ 318 ]. The results showed promising efficacy and safety for these patients.
Enzalutamide, a nonsteroidal antiandrogen, has been tested in a phase II study in patients with locally advanced or metastatic AR+ TNBC [ 319 ]. Enzalutamide demonstrated significant clinical activity and tolerability, warranting further investigation.
Abiraterone, a selective inhibitor of CYP17, has been evaluated in combination with prednisone in AR+ locally advanced or metastatic TNBC patients [ 320 ]. This combination was beneficial for 20% of the patients.
Several clinical trials are currently testing AR inhibitors alone or combined with other treatments for TNBC patients; expecting results between 2022 and 2027 (see Table 4 ).
4.3.3. Immunotherapy
Targeted antibodies.
The immune system plays a crucial role in BC development and progression. Tumor cells can escape the immune system by regulating T-cell activity leading to the inhibition of immune response [ 346 , 347 ]. Two principal biomarkers found in TNBC are associated with this bypass: the programmed cell death protein receptor (PD-1) and its ligand PDL-1, and the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) [ 348 ].
PD-1 is an immune checkpoint receptor expressed on the surface of activated T-cells. PDL-1, its ligand, is expressed on the surface of dendritic cells or macrophages. The interaction of PD-1 and PDL-1 inhibits T-cell response [ 349 ]. CTLA-4 is expressed on T-cells and inhibits T-cell activation by binding to CD80/CD86, leading to decreased immune response [ 350 ].
Atezolizumab, an anti-PDL-1 antibody, has demonstrated safety and efficacy in a phase I study for metastatic TNBC patients [ 351 ]. Based on these results, atezolizumab was tested in combination with nab-paclitaxel for unresectable locally advanced or metastatic TNBC in the phase III double-blinded placebo-controlled randomized Impassion130 study [ 321 ]. Atezolizumab plus nab-paclitaxel prolonged PFS and OS in both the intention-to-treat population and PDL1+ subgroup. Another double-blinded, randomized phase III study (Impassion031) compared atezolizumab in combination with nab-paclitaxel and anthracycline-based chemotherapy vs. placebo for early-stage TNBC [ 322 ]. This combination significantly improved pCR with an acceptable safety profile.
Durvalumab, another anti-PDL-1 antibody, has been tested in combination with an anthracycline taxane-based neoadjuvant therapy for early TNBC in the randomized phase II GeparNuevo study [ 323 ]. This combination increased pCR rate, particularly in patients pretreated with durvalumab monotherapy before chemotherapy. Another randomized phase II study, SAFIRO BREAST-IMMUNO, compared durvalumab to maintenance chemotherapy in a cohort including TNBC patients [ 324 ]. Results showed that durvalumab, as a single agent therapy, could improve outcomes in TNBC patients. A phase I study tested durvalumab in combination with multiple TNBC therapies: PARP inhibitor olaparib and VEGFR1-3 inhibitor cediranib for patients with recurrent cancers including TNBC [ 325 ]. This combination was well tolerated and showed preliminary antitumor activity in all of these patients.
The safety and efficacy of avelumab, another anti-PDL-1 antibody, was evaluated in the phase Ib JAVELIN study in patients with locally advanced or metastatic BC, including TNBC [ 326 ]. Avelumab showed an acceptable safety profile and clinical activity, particularly in tumors expressing PDL-1.
Pembrolizumab is an anti-PD-1 antibody that has been tested in multiple clinical trials. The phase Ib KEYNOTE-012 study demonstrated the safety and efficacy of pembrolizumab on advanced TNBC patients [ 352 ]. Based on these results, the phase II KEYNOTE-086 study evaluated pembrolizumab monotherapy for pretreated or non-pretreated metastatic TNBC patients [ 327 , 353 ]. Pembrolizumab monotherapy showed a manageable safety profile and durable antitumor activity for both pretreated and non-pretreated subgroups. The randomized open-labeled phase III KEYNOTE-119 trial compared pembrolizumab monotherapy to standard chemotherapy in metastatic TNBC [ 354 ]. Pembrolizumab monotherapy did not significantly improve OS compared to chemotherapy in these patients. These findings suggest that pembrolizumab should be investigated in a combinational approach rather than in monotherapy. Based on these results, pembrolizumab was tested in combination with chemotherapy (vs. placebo) for pretreated locally recurrent or metastatic TNBC patients in the phase III double-blinded randomized KEYNOTE-355 trial [ 328 ]. The combination of pembrolizumab plus chemotherapy significantly and clinically improved PFS compared to chemotherapy plus placebo. Pembrolizumab has also been evaluated for early TNBC as neoadjuvant therapy in combination with chemotherapy (vs. placebo) in the phase III KEYNOTE-522 trial [ 329 ]. The combination of pembrolizumab plus chemotherapy significantly improved pCR rate in these patients compared to placebo plus chemotherapy.
Tremelimumab is an anti-CTLA-4 antibody. A dose-escalation phase I study evaluating the safety and efficacy of tremelimumab in patients with metastatic BC showed good tolerability [ 330 ].
Vaccination is an emerging approach to prevent recurrence in high-risk BC patients. As mentioned earlier, TNBC is the most aggressive BC subtype with a higher risk of distant recurrence [ 331 ]. Thus, developing vaccines to prevent recurrence in TNBC patients is of great interest.
Takahashi et al. have developed a novel regimen of personalized peptide vaccination (PPV) based on the patient’s immune system to select vaccine antigens from a pool of peptide candidates [ 332 ]. They performed a phase II study where metastatic recurrent BC patients with prior chemotherapy and/or hormonal therapies received a series of personalized vaccines. This vaccination demonstrated safety, possible clinical benefit, and immune response, especially for TNBC patients [ 332 ]. A multicentered, randomized, double-blinded phase III study analyzed the effects of sialyl-TN keyhole limpet hemocyanin (STn-KLH) on metastatic BC patients [ 333 ]. STn-KLH consists of a synthetic STn, an epitope expressed in BC and associated with aggressive and metastatic tumors, and a high molecular weight protein carrier KLH [ 355 ]. Stn-KLH demonstrated good tolerability, but no benefits in time to progression (TTP) or survival were found. Thus, this vaccination is not recommended for metastatic BC patients [ 333 ].
PVX-410 is a multiple peptide vaccine that activates T-cell to target tumor cells and was developed to treat myeloma. A phase Ib/II study demonstrated the safety and immunogenicity in myeloma patients [ 356 ]. Based on these results, a PVX-410 vaccine is currently being tested to treat TNBC in multiple clinical trials (see Table 4 ).
Finding new treatments for TNBC is an ongoing challenge. The therapeutic strategies that have been described in this section offer great hope to treat TNBC patients. However, because TNBC is highly heterogeneous, it is difficult to find a single treatment efficient for all TNBC subtypes [ 228 ].
5. Conclusions
This review clearly demonstrates that the treatment of BC is complex and is constantly evolving with a large number of ongoing clinical trials on emerging therapies. Indeed, the BC molecular subtype will determine the personalized therapeutic approach, such as targeted treatments like endocrine therapy for HR+ BC or anti-HER2 therapy for HER2+ BC. These therapies have demonstrated their safety and efficacy in treating BC over the years. However, it is essential to go beyond these conventional treatments as BC is a complex disease and not all patients can benefit from personalized treatment. One of the major challenges in BC treatment is finding effective therapies to treat TNBC patients since conventional targeted therapies cannot be administered for this specific BC subtype, which has the worst survival outcomes.
Another important issue in BC treatment is the acquisition of treatment resistance. This is a common phenomenon for either endocrine therapy, anti-HER2 therapy, and chemotherapy.
Hence, understanding the mechanisms underlying drug resistance is a good strategy to develop novel treatments for BC. For example, the mTOR/PI3K/Akt pathway is involved in the mechanism of resistance in all BC molecular subtypes, and thus developing specific inhibitors targeting this pathway is a promising BC treatment approach.
Acknowledgments
The authors would also like to thank team members from the C.D. and F.D. research groups for their valuable assistance.
Abbreviation
Author contributions.
A.B. conceptualized and drafted the manuscript. F.D. and C.D. supervised the project. All authors did critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
This work was supported by the “Fond de recherche du Québec–Santé (FRQS)” associated with the Canadian Tumor Repository Network (CTRNet). Caroline Diorio is a senior Research Scholar from the FRSQ. Anna Burguin holds a Bourse d’excellence en recherche sur le cancer du sein—Faculté de médecine-Université Laval.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement.
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Joshi H., Press M.F. The Breast. Elsevier; Amsterdam, The Netherlands: 2018. [(accessed on 30 May 2021)]. Molecular Oncology of Breast Cancer; pp. 282–307.e5. Available online: https://www.sciencedirect.com/science/article/pii/B9780323359559000222 . [ Google Scholar ]
- 3. Gao J.J., Swain S.M. Luminal A Breast Cancer and Molecular Assays: A Review. Oncologist. 2018;23:556–565. doi: 10.1634/theoncologist.2017-0535. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Ades F., Zardavas D., Bozovic-Spasojevic I., Pugliano L., Fumagalli D., de Azambuja E., Viale G., Sotiriou C., Piccart M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Bergin A.R.T., Loi S. Triple-negative breast cancer: Recent treatment advances. F1000Research. 2019;8 doi: 10.12688/f1000research.18888.1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Female Breast Cancer Subtypes—Cancer Stat Facts. [(accessed on 31 May 2021)]; Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html .
- 8. Elston E.W., Ellis I.O. Method for grading breast cancer. J. Clin. Pathol. 1993;46:189–190. doi: 10.1136/jcp.46.2.189-b. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Amin M.B., Greene F.L., Edge S.B., Compton C.C., Gershenwald J.E., Brookland R.K., Meyer L., Gress D.M., Byrd D.R., Winchester D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging: The Eighth Edition AJCC Cancer Staging Manual. CA Cancer J. Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F. Breast cancer. Nat. Rev. Dis. Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Pisani P., Bray F., Parkin D.M. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int. J. Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Al-thoubaity F.K. Molecular classification of breast cancer: A retrospective cohort study. Ann. Med. Surg. 2020;49:44–48. doi: 10.1016/j.amsu.2019.11.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Hergueta-Redondo M., Palacios J., Cano A., Moreno-Bueno G. “New” molecular taxonomy in breast cancer. Clin. Transl. Oncol. 2008;10:777–785. doi: 10.1007/s12094-008-0290-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Engstrøm M.J., Opdahl S., Hagen A.I., Romundstad P.R., Akslen L.A., Haugen O.A., Vatten L.J., Bofin A.M. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res. Treat. 2013;140:463–473. doi: 10.1007/s10549-013-2647-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Hennigs A., Riedel F., Gondos A., Sinn P., Schirmacher P., Marmé F., Jäger D., Kauczor H.-U., Stieber A., Lindel K., et al. Prognosis of breast cancer molecular subtypes in routine clinical care: A large prospective cohort study. BMC Cancer. 2016;16:734. doi: 10.1186/s12885-016-2766-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Fragomeni S.M., Sciallis A., Jeruss J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018;27:95–120. doi: 10.1016/j.soc.2017.08.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Nofech-Mozes S., Trudeau M., Kahn H.K., Dent R., Rawlinson E., Sun P., Narod S.A., Hanna W.M. Patterns of recurrence in the basal and non-basal subtypes of triple-negative breast cancers. Breast Cancer Res. Treat. 2009;118:131–137. doi: 10.1007/s10549-008-0295-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Schnitt S.J., Moran M.S., Giuliano A.E. Lumpectomy Margins for Invasive Breast Cancer and Ductal Carcinoma in Situ: Current Guideline Recommendations, Their Implications, and Impact. JCO. 2020;38:2240–2245. doi: 10.1200/JCO.19.03213. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R., Jeong J.-H., Wolmark N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Fisher B., Montague E., Redmond C., Deutsch M., Brown G.R., Zauber A., Hanson W.F., Wong A. Findings from NSABP Protocol No. B-04-comparison of radical mastectomy with alternative treatments for primary breast cancer. I. Radiation compliance and its relation to treatment outcome. Cancer. 1980;46:1–13. doi: 10.1002/1097-0142(19800701)46:1<1::AID-CNCR2820460102>3.0.CO;2-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Sener S.F. Advances in axillary surgery for breast cancer 2019. J. Surg. Oncol. 2019 doi: 10.1002/jso.25591. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Grubbé E.H. Priority in the Therapeutic Use of X-rays. Radiology. 1933;21:156–162. doi: 10.1148/21.2.156. [ DOI ] [ Google Scholar ]
- 23. Boyages J. Radiation therapy and early breast cancer: Current controversies. Med. J. Aust. 2017;207:216–222. doi: 10.5694/mja16.01020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M., Cutter D., Davies C., Ewertz M., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Bartelink H., Maingon P., Poortmans P., Weltens C., Fourquet A., Jager J., Schinagl D., Oei B., Rodenhuis C., Horiot J.-C., et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47–56. doi: 10.1016/S1470-2045(14)71156-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Whelan T.J., Olivotto I.A., Parulekar W.R., Ackerman I., Chua B.H., Nabid A., Vallis K.A., White J.R., Rousseau P., Fortin A., et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Cheng Y., Nie X., Ji C., Lin X., Liu L., Chen X., Yao H., Wu S. Long-Term Cardiovascular Risk After Radiotherapy in Women With Breast Cancer. JAHA. 2017;6 doi: 10.1161/JAHA.117.005633. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Taylor C.W., Kirby A.M. Cardiac Side-effects From Breast Cancer Radiotherapy. Clin. Oncol. 2015;27:621–629. doi: 10.1016/j.clon.2015.06.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Delaney G., Jacob S., Featherstone C., Barton M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Gilreath C., Boerma M., Qin Z., Hudson M.K., Wang S. The Hypoxic Microenvironment of Breast Cancer Cells Promotes Resistance in Radiation Therapy. Front. Oncol. 2020;10:629422. doi: 10.3389/fonc.2020.629422. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Krishnamachary B., Penet M.-F., Nimmagadda S., Mironchik Y., Raman V., Solaiyappan M., Semenza G.L., Pomper M.G., Bhujwalla Z.M. Hypoxia Regulates CD44 and Its Variant Isoforms through HIF-1α in Triple Negative Breast Cancer. PLoS ONE. 2012;7:e44078. doi: 10.1371/journal.pone.0044078. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Kim J., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Jiang B.H., Agani F., Passaniti A., Semenza G.L. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: Involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328–5335. [ PubMed ] [ Google Scholar ]
- 35. Blancher C., Moore J.W., Talks K.L., Houlbrook S., Harris A.L. Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2000;60:7106–7113. [ PubMed ] [ Google Scholar ]
- 36. Qi X.S., Pajonk F., McCloskey S., Low D.A., Kupelian P., Steinberg M., Sheng K. Radioresistance of the breast tumor is highly correlated to its level of cancer stem cell and its clinical implication for breast irradiation. Radiother. Oncol. 2017;124:455–461. doi: 10.1016/j.radonc.2017.08.019. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Lagadec C., Vlashi E., Della Donna L., Meng Y., Dekmezian C., Kim K., Pajonk F. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010;12:R13. doi: 10.1186/bcr2479. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Lock F.E., McDonald P.C., Lou Y., Serrano I., Chafe S.C., Ostlund C., Aparicio S., Winum J.-Y., Supuran C.T., Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32:5210–5219. doi: 10.1038/onc.2012.550. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. He M.Y., Rancoule C., Rehailia-Blanchard A., Espenel S., Trone J.-C., Bernichon E., Guillaume E., Vallard A., Magné N. Radiotherapy in triple-negative breast cancer: Current situation and upcoming strategies. Crit. Rev. Oncol. Hematol. 2018;131:96–101. doi: 10.1016/j.critrevonc.2018.09.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Nabholtz J.-M., Gligorov J. The role of taxanes in the treatment of breast cancer. Expert Opin. Pharmacother. 2005;6:1073–1094. doi: 10.1517/14656566.6.7.1073. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Penel N., Adenis A., Bocci G. Cyclophosphamide-based metronomic chemotherapy: After 10 years of experience, where do we stand and where are we going? Crit. Rev. Oncol. Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57:727–741. doi: 10.1016/S0006-2952(98)00307-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Moos P.J., Fitzpatrick F.A. Taxanes propagate apoptosis via two cell populations with distinctive cytological and molecular traits. Cell Growth Differ. 1998;9:687–697. [ PubMed ] [ Google Scholar ]
- 44. Chira C., Kirova Y.M., Liem X., Campana F., Peurien D., Amessis M., Fournier-Bidoz N., Pierga J.-Y., Dendale R., Bey P., et al. Helical Tomotherapy for Inoperable Breast Cancer: A New Promising Tool. BioMed Res. Int. 2013;2013:264306. doi: 10.1155/2013/264306. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Montemurro F., Nuzzolese I., Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opin. Pharmacother. 2020;21:1071–1082. doi: 10.1080/14656566.2020.1746273. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Broët P., Scholl S.M., de la Rochefordière A., Fourquet A., Moreau T., De Rycke Y., Asselain B., Pouillart P. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: An updated analysis of a randomized trial. Breast Cancer Res. Treat. 1999;58:151–156. doi: 10.1023/A:1006339918798. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. van der Hage J.A., van de Velde C.J., Julien J.P., Tubiana-Hulin M., Vandervelden C., Duchateau L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J. Clin. Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Fisher B., Bryant J., Wolmark N., Mamounas E., Brown A., Fisher E.R., Wickerham D.L., Begovic M., DeCillis A., Robidoux A., et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. von Minckwitz G., Rezai M., Tesch H., Huober J., Gerber B., Zahm D.M., Hilfrich J., Costa S.D., Dubsky P., Blohmer J.U., et al. Zoledronate for patients with invasive residual disease after anthracyclines-taxane-based chemotherapy for early breast cancer—The Phase III NeoAdjuvant Trial Add-oN (NaTaN) study (GBG 36/ABCSG 29) Eur. J. Cancer. 2016;64:12–21. doi: 10.1016/j.ejca.2016.05.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Gonzalez-Angulo A.M., Lei X., Alvarez R.H., Green M.C., Murray J.L., Valero V., Koenig K.B., Ibrahim N.K., Litton J.K., Nair L., et al. Phase II Randomized Study of Ixabepilone Versus Observation in Patients With Significant Residual Disease After Neoadjuvant Systemic Therapy for HER2-Negative Breast Cancer. Clin. Breast Cancer. 2015;15:325–331. doi: 10.1016/j.clbc.2015.03.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R., Davies C., Godwin J., Gray R., Pan H.C., Clarke M., Cutter D., Darby S., McGale P., et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Citron M.L., Berry D.A., Cirrincione C., Hudis C., Winer E.P., Gradishar W.J., Davidson N.E., Martino S., Livingston R., Ingle J.N., et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Sparano J.A., Wang M., Martino S., Jones V., Perez E.A., Saphner T., Wolff A.C., Sledge G.W., Wood W.C., Davidson N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Berry D.A., Cirrincione C., Henderson I.C., Citron M.L., Budman D.R., Goldstein L.J., Martino S., Perez E.A., Muss H.B., Norton L., et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Paik S., Tang G., Shak S., Kim C., Baker J., Kim W., Cronin M., Baehner F.L., Watson D., Bryant J., et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F., Geyer C.E., Dees E.C., Perez E.A., Olson J.A., et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Anampa J., Makower D., Sparano J.A. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015;13:195. doi: 10.1186/s12916-015-0439-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Cardoso F., Senkus E., Costa A., Papadopoulos E., Aapro M., André F., Harbeck N., Aguilar Lopez B., Barrios C.H., Bergh J., et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) Ann. Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Tao J.J., Visvanathan K., Wolff A.C. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast. 2015;24(Suppl 2):S149–S153. doi: 10.1016/j.breast.2015.07.035. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Coley H.M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 2008;34:378–390. doi: 10.1016/j.ctrv.2008.01.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Fojo T., Menefee M. Mechanisms of multidrug resistance: The potential role of microtubule-stabilizing agents. Ann. Oncol. 2007;18:v3–v8. doi: 10.1093/annonc/mdm172. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Faneyte I.F., Kristel P.M.P., Maliepaard M., Scheffer G.L., Scheper R.J., Schellens J.H.M., van de Vijver M.J. Expression of the breast cancer resistance protein in breast cancer. Clin. Cancer Res. 2002;8:1068–1074. [ PubMed ] [ Google Scholar ]
- 63. Kamath K., Wilson L., Cabral F., Jordan M.A. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J. Biol. Chem. 2005;280:12902–12907. doi: 10.1074/jbc.M414477200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Rouzier R., Rajan R., Wagner P., Hess K.R., Gold D.L., Stec J., Ayers M., Ross J.S., Zhang P., Buchholz T.A., et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Sládek N.E. Aldehyde dehydrogenase-mediated cellular relative insensitivity to the oxazaphosphorines. Curr. Pharm. Des. 1999;5:607–625. [ PubMed ] [ Google Scholar ]
- 66. Zhang J., Tian Q., Chan S.Y., Duan W., Zhou S. Insights into oxazaphosphorine resistance and possible approaches to its circumvention. Drug Resist. Updates. 2005;8:271–297. doi: 10.1016/j.drup.2005.08.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Howlader N., Altekruse S.F., Li C.I., Chen V.W., Clarke C.A., Ries L.A.G., Cronin K.A. US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju055. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. El Sayed R., El Jamal L., El Iskandarani S., Kort J., Abdel Salam M., Assi H. Endocrine and Targeted Therapy for Hormone-Receptor-Positive, HER2-Negative Advanced Breast Cancer: Insights to Sequencing Treatment and Overcoming Resistance Based on Clinical Trials. Front. Oncol. 2019;9:510. doi: 10.3389/fonc.2019.00510. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Brzozowski A.M., Pike A.C.W., Dauter Z., Hubbard R.E., Bonn T., Engström O., Öhman L., Greene G.L., Gustafsson J.-Å., Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Björnström L., Sjöberg M. Mechanisms of Estrogen Receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Mol. Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Pagani O., Regan M.M., Walley B.A., Fleming G.F., Colleoni M., Láng I., Gomez H.L., Tondini C., Burstein H.J., Perez E.A., et al. Adjuvant Exemestane with Ovarian Suppression in Premenopausal Breast Cancer. N. Engl. J. Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Mustacchi G., Ceccherini R., Milani S., Pluchinotta A., De Matteis A., Maiorino L., Farris A., Scanni A., Sasso F., Italian Cooperative Group GRETA Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: Long-term results of the phase III randomized controlled multicenter GRETA trial. Ann. Oncol. 2003;14:414–420. doi: 10.1093/annonc/mdg117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Robertson J.F., Todd J.H., Ellis I.O., Elston C.W., Blamey R.W. Comparison of mastectomy with tamoxifen for treating elderly patients with operable breast cancer. BMJ. 1988;297:511–514. doi: 10.1136/bmj.297.6647.511. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Gazet J.C., Ford H.T., Coombes R.C., Bland J.M., Sutcliffe R., Quilliam J., Lowndes S. Prospective randomized trial of tamoxifen vs. surgery in elderly patients with breast cancer. Eur. J. Surg. Oncol. 1994;20:207–214. [ PubMed ] [ Google Scholar ]
- 76. Gershanovich M., Garin A., Baltina D., Kurvet A., Kangas L., Ellmén J., Eastern European Study Group A phase III comparison of two toremifene doses to tamoxifen in postmenopausal women with advanced breast cancer. Breast Cancer Res. Treat. 1997;45:251–262. doi: 10.1023/A:1005891506092. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Pyrhönen S., Valavaara R., Modig H., Pawlicki M., Pienkowski T., Gundersen S., Bauer J., Westman G., Lundgren S., Blanco G., et al. Comparison of toremifene and tamoxifen in post-menopausal patients with advanced breast cancer: A randomized double-blind, the “nordic” phase III study. Br. J. Cancer. 1997;76:270–277. doi: 10.1038/bjc.1997.375. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Ye Q., Zhai Z. Toremifene and tamoxifen have similar efficacy in the treatment of patients with breast cancer: A meta-analysis of randomized trials. Mol. Biol. Rep. 2014;41:751–756. doi: 10.1007/s11033-013-2914-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Komm B.S., Kharode Y.P., Bodine P.V.N., Harris H.A., Miller C.P., Lyttle C.R. Bazedoxifene Acetate: A Selective Estrogen Receptor Modulator with Improved Selectivity. Endocrinology. 2005;146:3999–4008. doi: 10.1210/en.2005-0030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Vogel V.G. Effects of Tamoxifen vs. Raloxifene on the Risk of Developing Invasive Breast Cancer and Other Disease OutcomesThe NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727. doi: 10.1001/jama.295.23.joc60074. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Vogel V.G., Costantino J.P., Wickerham D.L., Cronin W.M., Cecchini R.S., Atkins J.N., Bevers T.B., Fehrenbacher L., Pajon E.R., Wade J.L., et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing Breast Cancer. Cancer Prev. Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Ellis A.J., Hendrick V.M., Williams R., Komm B.S. Selective estrogen receptor modulators in clinical practice: A safety overview. Expert Opin. Drug Saf. 2015;14:921–934. doi: 10.1517/14740338.2015.1014799. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Ring A., Dowsett M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Sharma D., Blum J., Yang X., Beaulieu N., Macleod A.R., Davidson N.E. Release of Methyl CpG Binding Proteins and Histone Deacetylase 1 from the Estrogen Receptor α (ER) Promoter upon Reactivation in ER-Negative Human Breast Cancer Cells. Mol. Endocrinol. 2005;19:1740–1751. doi: 10.1210/me.2004-0011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Ellis M.J., Tao Y., Luo J., A’Hern R., Evans D.B., Bhatnagar A.S., Chaudri Ross H.A., von Kameke A., Miller W.R., Smith I., et al. Outcome Prediction for Estrogen Receptor-Positive Breast Cancer Based on Postneoadjuvant Endocrine Therapy Tumor Characteristics. J. Natl. Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Toy W., Shen Y., Won H., Green B., Sakr R.A., Will M., Li Z., Gala K., Fanning S., King T.A., et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Barone I., Brusco L., Fuqua S.A.W. Estrogen Receptor Mutations and Changes in Downstream Gene Expression and Signaling. Clin. Cancer Res. 2010;16:2702–2708. doi: 10.1158/1078-0432.CCR-09-1753. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Clarke R., Liu M.C., Bouker K.B., Gu Z., Lee R.Y., Zhu Y., Skaar T.C., Gomez B., O’Brien K., Wang Y., et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Manavathi B., Samanthapudi V.S.K., Gajulapalli V.N.R. Estrogen receptor coregulators and pioneer factors: The orchestrators of mammary gland cell fate and development. Front. Cell Dev. Biol. 2014;2 doi: 10.3389/fcell.2014.00034. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Osborne C.K., Bardou V., Hopp T.A., Chamness G.C., Hilsenbeck S.G., Fuqua S.A.W., Wong J., Allred D.C., Clark G.M., Schiff R. Role of the Estrogen Receptor Coactivator AIB1 (SRC-3) and HER-2/neu in Tamoxifen Resistance in Breast Cancer. J. Natl. Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Su Q., Hu S., Gao H., Ma R., Yang Q., Pan Z., Wang T., Li F. Role of AIB1 for Tamoxifen Resistance in Estrogen Receptor-Positive Breast Cancer Cells. Oncology. 2008;75:159–168. doi: 10.1159/000159267. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Wakeling A.E., Bowler J. Novel antioestrogens without partial agonist activity. J. Steroid Biochem. 1988;31:645–653. doi: 10.1016/0022-4731(88)90014-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Dauvois S., White R., Parker M.G. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J. Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Wakeling A.E. Steroidal pure antiestrogens. In: Lippman M.E., Dickson R.B., editors. Regulatory Mechanisms in Breast Cancer. Volume 53. Springer; Boston, MA, USA: 1991. pp. 239–257. Cancer Treatment and Research. [ PubMed ] [ Google Scholar ]
- 95. Wakeling A.E., Dukes M., Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [ PubMed ] [ Google Scholar ]
- 96. Lee C.I., Goodwin A., Wilcken N. Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD011093.pub2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Leo A.D., Jerusalem G., Petruzelka L., Torres R., Bondarenko I.N., Khasanov R., Verhoeven D., Pedrini J.L., Smirnova I., Lichinitser M.R., et al. Final Overall Survival: Fulvestrant 500 mg vs. 250 mg in the Randomized CONFIRM Trial. J. Natl. Cancer Inst. 2014;106:djt337. doi: 10.1093/jnci/djt337. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Robertson J.F.R., Bondarenko I.M., Trishkina E., Dvorkin M., Panasci L., Manikhas A., Shparyk Y., Cardona-Huerta S., Cheung K.-L., Philco-Salas M.J., et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. NCCN Clinical Practice Guidelines in Oncology. Version 5. [(accessed on 30 June 2021)];2021 Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .
- 100. Bergh J., Jönsson P.-E., Lidbrink E.K., Trudeau M., Eiermann W., Brattström D., Lindemann J.P.O., Wiklund F., Henriksson R. FACT: An Open-Label Randomized Phase III Study of Fulvestrant and Anastrozole in Combination Compared With Anastrozole Alone As First-Line Therapy for Patients With Receptor-Positive Postmenopausal Breast Cancer. JCO. 2012;30:1919–1925. doi: 10.1200/JCO.2011.38.1095. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Johnston S.R., Kilburn L.S., Ellis P., Dodwell D., Cameron D., Hayward L., Im Y.-H., Braybrooke J.P., Brunt A.M., Cheung K.-L., et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): A composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14:989–998. doi: 10.1016/S1470-2045(13)70322-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Jeselsohn R., Yelensky R., Buchwalter G., Frampton G., Meric-Bernstam F., Gonzalez-Angulo A.M., Ferrer-Lozano J., Perez-Fidalgo J.A., Cristofanilli M., Gómez H., et al. Emergence of Constitutively Active Estrogen Receptor-α Mutations in Pretreated Advanced Estrogen Receptor–Positive Breast Cancer. Clin. Cancer Res. 2014;20:1757–1767. doi: 10.1158/1078-0432.CCR-13-2332. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Fribbens C., O’Leary B., Kilburn L., Hrebien S., Garcia-Murillas I., Beaney M., Cristofanilli M., Andre F., Loi S., Loibl S., et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor–Positive Advanced Breast Cancer. JCO. 2016;34:2961–2968. doi: 10.1200/JCO.2016.67.3061. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Stemke-Hale K., Gonzalez-Angulo A.M., Lluch A., Neve R.M., Kuo W.-L., Davies M., Carey M., Hu Z., Guan Y., Sahin A., et al. An Integrative Genomic and Proteomic Analysis of PIK3CA, PTEN, and AKT Mutations in Breast Cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Sengupta S., Peterson T.R., Sabatini D.M. Regulation of the mTOR Complex 1 Pathway by Nutrients, Growth Factors, and Stress. Mol. Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Beeram M., Tan Q.-T.N., Tekmal R.R., Russell D., Middleton A., deGraffenried L.A. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann. Oncol. 2007;18:1323–1328. doi: 10.1093/annonc/mdm170. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Zhang Y., Moerkens M., Ramaiahgari S., de Bont H., Price L., Meerman J., van de Water B. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13:R52. doi: 10.1186/bcr2883. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Akhtar M., Wright J.N., Lee-Robichaud P. A review of mechanistic studies on aromatase (CYP19) and 17α-hydroxylase-17,20-lyase (CYP17) J. Steroid Biochem. Mol. Biol. 2011;125:2–12. doi: 10.1016/j.jsbmb.2010.11.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Miller W.R., O’Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50:537–548. doi: 10.1016/0039-128X(87)90037-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Brueggemeier R.W., Hackett J.C., Diaz-Cruz E.S. Aromatase Inhibitors in the Treatment of Breast Cancer. Endocr. Rev. 2005;26:331–345. doi: 10.1210/er.2004-0015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Bulun S.E., Price T.M., Aitken J., Mahendroo M.S., Simpson E.R. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J. Clin. Endocrinol. Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Chen S., Besman M.J., Sparkes R.S., Zollman S., Klisak I., Mohandas T., Hall P.F., Shively J.E. Human Aromatase: cDNA Cloning, Southern Blot Analysis, and Assignment of the Gene to Chromosome 15. DNA. 1988;7:27–38. doi: 10.1089/dna.1988.7.27. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Thompson E.A., Siiteri P.K. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J. Biol. Chem. 1974;249:5364–5372. doi: 10.1016/S0021-9258(20)79735-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Regan M.M., Neven P., Giobbie-Hurder A., Goldhirsch A., Ejlertsen B., Mauriac L., Forbes J.F., Smith I., Láng I., Wardley A., et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: The BIG 1-98 randomised clinical trial at 81 years median follow-up. Lancet Oncol. 2011;12:1101–1108. doi: 10.1016/S1470-2045(11)70270-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Cuzick J., Sestak I., Baum M., Buzdar A., Howell A., Dowsett M., Forbes J.F. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. van de Velde C.J., Rea D., Seynaeve C., Putter H., Hasenburg A., Vannetzel J.-M., Paridaens R., Markopoulos C., Hozumi Y., Hille E.T., et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): A randomised phase 3 trial. Lancet. 2011;377:321–331. doi: 10.1016/S0140-6736(10)62312-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Dubsky P.C., Jakesz R., Mlineritsch B., Pöstlberger S., Samonigg H., Kwasny W., Tausch C., Stöger H., Haider K., Fitzal F., et al. Tamoxifen and Anastrozole As a Sequencing Strategy: A Randomized Controlled Trial in Postmenopausal Patients With Endocrine-Responsive Early Breast Cancer From the Austrian Breast and Colorectal Cancer Study Group. JCO. 2012;30:722–728. doi: 10.1200/JCO.2011.36.8993. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Goss P.E., Ingle J.N., Martino S., Robert N.J., Muss H.B., Piccart M.J., Castiglione M., Tu D., Shepherd L.E., Pritchard K.I., et al. A Randomized Trial of Letrozole in Postmenopausal Women after Five Years of Tamoxifen Therapy for Early-Stage Breast Cancer. N. Engl. J. Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Jakesz R., Greil R., Gnant M., Schmid M., Kwasny W., Kubista E., Mlineritsch B., Tausch C., Stierer M., Hofbauer F., et al. Extended Adjuvant Therapy With Anastrozole Among Postmenopausal Breast Cancer Patients: Results From the Randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J. Natl. Cancer Inst. 2007;99:1845–1853. doi: 10.1093/jnci/djm246. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Mendelsohn M.E., Karas R.H. The Protective Effects of Estrogen on the Cardiovascular System. N. Engl. J. Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Braithwaite R.S., Chlebowski R.T., Lau J., George S., Hess R., Col N.F. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J. Gen. Intern. Med. 2003;18:937–947. doi: 10.1046/j.1525-1497.2003.20724.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 122. Winer E.P. Optimizing Endocrine Therapy for Breast Cancer. JCO. 2005;23:1609–1610. doi: 10.1200/JCO.2005.01.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Ma C.X., Reinert T., Chmielewska I., Ellis M.J. Mechanisms of aromatase inhibitor resistance. Nat. Rev. Cancer. 2015;15:261–275. doi: 10.1038/nrc3920. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. The Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Finn R., Crown J., Lang I., Boer K., Bondarenko I., Kulyk S., Ettl J., Patel R., Pinter T., Schmidt M., et al. Abstract S1-6: Results of a Randomized Phase 2 Study of PD 0332991, a Cyclin-Dependent Kinase (CDK) 4/6 Inhibitor, in Combination with Letrozole vs. Letrozole Alone for First-Line Treatment of ER+/HER2− Advanced Breast Cancer (BC). General Session Abstracts. American Association for Cancer Research; Philadelphia, PA, USA: 2012. [(accessed on 8 March 2021)]. pp. S1–S6. Available online: http://cancerres.aacrjournals.org/lookup/doi/10.1158/0008-5472.SABCS12-S1-6 . [ Google Scholar ]
- 126. Finn R.S., Crown J.P., Lang I., Boer K., Bondarenko I.M., Kulyk S.O., Ettl J., Patel R., Pinter T., Schmidt M., et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Szostakowska M., Trębińska-Stryjewska A., Grzybowska E.A., Fabisiewicz A. Resistance to endocrine therapy in breast cancer: Molecular mechanisms and future goals. Breast Cancer Res. Treat. 2019;173:489–497. doi: 10.1007/s10549-018-5023-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 128. Slamon D., Clark G., Wong S., Levin W., Ullrich A., McGuire W. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 129. Press M.F., Pike M.C., Chazin V.R., Hung G., Udove J.A., Markowicz M., Danyluk J., Godolphin W., Sliwkowski M., Akita R. Her-2/neu expression in node-negative breast cancer: Direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–4970. [ PubMed ] [ Google Scholar ]
- 130. Goutsouliak K., Veeraraghavan J., Sethunath V., De Angelis C., Osborne C.K., Rimawi M.F., Schiff R. Towards personalized treatment for early stage HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 2020;17:233–250. doi: 10.1038/s41571-019-0299-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Carter P., Presta L., Gorman C.M., Ridgway J.B., Henner D., Wong W.L., Rowland A.M., Kotts C., Carver M.E., Shepard H.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., Eiermann W., Wolter J., Pegram M., et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. Junttila T.T., Akita R.W., Parsons K., Fields C., Lewis Phillips G.D., Friedman L.S., Sampath D., Sliwkowski M.X. Ligand-Independent HER2/HER3/PI3K Complex Is Disrupted by Trastuzumab and Is Effectively Inhibited by the PI3K Inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Molina M.A., Codony-Servat J., Albanell J., Rojo F., Arribas J., Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [ PubMed ] [ Google Scholar ]
- 135. Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [ DOI ] [ PubMed ] [ Google Scholar ]
- 136. Perez E.A., Romond E.H., Suman V.J., Jeong J.-H., Davidson N.E., Geyer C.E., Martino S., Mamounas E.P., Kaufman P.A., Wolmark N. Four-Year Follow-Up of Trastuzumab Plus Adjuvant Chemotherapy for Operable Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Joint Analysis of Data From NCCTG N9831 and NSABP B-31. JCO. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 137. Perez E.A., Suman V.J., Davidson N.E., Gralow J.R., Kaufman P.A., Visscher D.W., Chen B., Ingle J.N., Dakhil S.R., Zujewski J., et al. Sequential Versus Concurrent Trastuzumab in Adjuvant Chemotherapy for Breast Cancer. JCO. 2011;29:4491–4497. doi: 10.1200/JCO.2011.36.7045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. Gianni L., Dafni U., Gelber R.D., Azambuja E., Muehlbauer S., Goldhirsch A., Untch M., Smith I., Baselga J., Jackisch C., et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 139. Iqbal N., Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014;2014:1–9. doi: 10.1155/2014/852748. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 140. Capelan M., Pugliano L., De Azambuja E., Bozovic I., Saini K.S., Sotiriou C., Loi S., Piccart-Gebhart M.J. Pertuzumab: New hope for patients with HER2-positive breast cancer. Ann. Oncol. 2013;24:273–282. doi: 10.1093/annonc/mds328. [ DOI ] [ PubMed ] [ Google Scholar ]
- 141. Scheuer W., Friess T., Burtscher H., Bossenmaier B., Endl J., Hasmann M. Strongly Enhanced Antitumor Activity of Trastuzumab and Pertuzumab Combination Treatment on HER2-Positive Human Xenograft Tumor Models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Baselga J., Gelmon K.A., Verma S., Wardley A., Conte P., Miles D., Bianchi G., Cortes J., McNally V.A., Ross G.A., et al. Phase II Trial of Pertuzumab and Trastuzumab in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer That Progressed During Prior Trastuzumab Therapy. JCO. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 143. Baselga J., Cortés J., Kim S.-B., Im S.-A., Hegg R., Im Y.-H., Roman L., Pedrini J.L., Pienkowski T., Knott A., et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Swain S.M., Kim S.-B., Cortés J., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.-M., Schneeweiss A., Knott A., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 145. Swain S.M., Baselga J., Kim S.-B., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.-M., Schneeweiss A., Heeson S., et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Hurvitz S.A., Martin M., Symmans W.F., Jung K.H., Huang C.-S., Thompson A.M., Harbeck N., Valero V., Stroyakovskiy D., Wildiers H., et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–126. doi: 10.1016/S1470-2045(17)30716-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 147. van Ramshorst M.S., van der Voort A., van Werkhoven E.D., Mandjes I.A., Kemper I., Dezentjé V.O., Oving I.M., Honkoop A.H., Tick L.W., van de Wouw A.J., et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1630–1640. doi: 10.1016/S1470-2045(18)30570-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Swain S.M., Ewer M.S., Viale G., Delaloge S., Ferrero J.-M., Verrill M., Colomer R., Vieira C., Werner T.L., Douthwaite H., et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018;29:646–653. doi: 10.1093/annonc/mdx773. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 149. Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Hegg R., Tausch C., Seo J.H., Tsai Y.-F., Ratnayake J., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann. Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Ng C.M., Lum B.L., Gimenez V., Kelsey S., Allison D. Rationale for Fixed Dosing of Pertuzumab in Cancer Patients Based on Population Pharmacokinetic Analysis. Pharm Res. 2006;23:1275–1284. doi: 10.1007/s11095-006-0205-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 151. Rimawi M.F., Schiff R., Osborne C.K. Targeting HER2 for the Treatment of Breast Cancer. Annu. Rev. Med. 2015;66:111–128. doi: 10.1146/annurev-med-042513-015127. [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Zagozdzon R., Gallagher W.M., Crown J. Truncated HER2: Implications for HER2-targeted therapeutics. Drug Discov. Today. 2011;16:810–816. doi: 10.1016/j.drudis.2011.06.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 153. Lu Y., Zi X., Zhao Y., Mascarenhas D., Pollak M. Insulin-Like Growth Factor-I Receptor Signaling and Resistance to Trastuzumab (Herceptin) J. Natl. Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [ DOI ] [ PubMed ] [ Google Scholar ]
- 154. Shattuck D.L., Miller J.K., Carraway K.L., Sweeney C. Met Receptor Contributes to Trastuzumab Resistance of Her2-Overexpressing Breast Cancer Cells. Cancer Res. 2008;68:1471–1477. doi: 10.1158/0008-5472.CAN-07-5962. [ DOI ] [ PubMed ] [ Google Scholar ]
- 155. Agarwal S., Zerillo C., Kolmakova J., Christensen J.G., Harris L.N., Rimm D.L., DiGiovanna M.P., Stern D.F. Association of constitutively activated hepatocyte growth factor receptor (Met) with resistance to a dual EGFR/Her2 inhibitor in non-small-cell lung cancer cells. Br. J. Cancer. 2009;100:941–949. doi: 10.1038/sj.bjc.6604937. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 156. Davis N.M., Sokolosky M., Stadelman K., Abrams S.L., Libra M., Candido S., Nicoletti F., Polesel J., Maestro R., D’Assoro A., et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: Possibilities for therapeutic intervention. Oncotarget. 2014;5:4603–4650. doi: 10.18632/oncotarget.2209. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 157. Berns K., Horlings H.M., Hennessy B.T., Madiredjo M., Hijmans E.M., Beelen K., Linn S.C., Gonzalez-Angulo A.M., Stemke-Hale K., Hauptmann M., et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 158. Kataoka Y., Mukohara T., Shimada H., Saijo N., Hirai M., Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann. Oncol. 2010;21:255–262. doi: 10.1093/annonc/mdp304. [ DOI ] [ PubMed ] [ Google Scholar ]
- 159. Hartmann J., Haap M., Kopp H.-G., Lipp H.-P. Tyrosine Kinase Inhibitors—A Review on Pharmacology, Metabolism and Side Effects. CDM. 2009;10:470–481. doi: 10.2174/138920009788897975. [ DOI ] [ PubMed ] [ Google Scholar ]
- 160. Montemurro F., Valabrega G., Aglietta M. Lapatinib: A dual inhibitor of EGFR and HER2 tyrosine kinase activity. Expert Opin. Biol. Ther. 2007;7:257–268. doi: 10.1517/14712598.7.2.257. [ DOI ] [ PubMed ] [ Google Scholar ]
- 161. Guan Z., Xu B., DeSilvio M.L., Shen Z., Arpornwirat W., Tong Z., Lorvidhaya V., Jiang Z., Yang J., Makhson A., et al. Randomized Trial of Lapatinib Versus Placebo Added to Paclitaxel in the Treatment of Human Epidermal Growth Factor Receptor 2–Overexpressing Metastatic Breast Cancer. JCO. 2013;31:1947–1953. doi: 10.1200/JCO.2011.40.5241. [ DOI ] [ PubMed ] [ Google Scholar ]
- 162. Di Leo A., Gomez H.L., Aziz Z., Zvirbule Z., Bines J., Arbushites M.C., Guerrera S.F., Koehler M., Oliva C., Stein S.H., et al. Phase III, Double-Blind, Randomized Study Comparing Lapatinib Plus Paclitaxel With Placebo Plus Paclitaxel As First-Line Treatment for Metastatic Breast Cancer. JCO. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 163. Johnston S., Pippen J., Pivot X., Lichinitser M., Sadeghi S., Dieras V., Gomez H.L., Romieu G., Manikhas A., Kennedy M.J., et al. Lapatinib Combined With Letrozole Versus Letrozole and Placebo As First-Line Therapy for Postmenopausal Hormone Receptor–Positive Metastatic Breast Cancer. JCO. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [ DOI ] [ PubMed ] [ Google Scholar ]
- 164. Guarneri V., Frassoldati A., Bottini A., Cagossi K., Bisagni G., Sarti S., Ravaioli A., Cavanna L., Giardina G., Musolino A., et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: Results of the randomized phase II CHER-LOB study. J. Clin. Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Goss P.E., Smith I.E., O’Shaughnessy J., Ejlertsen B., Kaufmann M., Boyle F., Buzdar A.U., Fumoleau P., Gradishar W., Martin M., et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: A randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:88–96. doi: 10.1016/S1470-2045(12)70508-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 166. Rabindran S.K., Discafani C.M., Rosfjord E.C., Baxter M., Floyd M.B., Golas J., Hallett W.A., Johnson B.D., Nilakantan R., Overbeek E., et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [ DOI ] [ PubMed ] [ Google Scholar ]
- 167. Chan A., Delaloge S., Holmes F.A., Moy B., Iwata H., Harvey V.J., Robert N.J., Silovski T., Gokmen E., von Minckwitz G., et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 168. Martin M., Holmes F.A., Ejlertsen B., Delaloge S., Moy B., Iwata H., von Minckwitz G., Chia S.K.L., Mansi J., Barrios C.H., et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 169. Li X., Yang C., Wan H., Zhang G., Feng J., Zhang L., Chen X., Zhong D., Lou L., Tao W., et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur. J. Pharm. Sci. 2017;110:51–61. doi: 10.1016/j.ejps.2017.01.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 170. Xuhong J.-C., Qi X.-W., Zhang Y., Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am. J. Cancer Res. 2019;9:2103–2119. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 171. Blair H.A. Pyrotinib: First Global Approval. Drugs. 2018;78:1751–1755. doi: 10.1007/s40265-018-0997-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 172. D’Amato V., Raimondo L., Formisano L., Giuliano M., De Placido S., Rosa R., Bianco R. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat. Rev. 2015;41:877–883. doi: 10.1016/j.ctrv.2015.08.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 173. Garrett J.T., Olivares M.G., Rinehart C., Granja-Ingram N.D., Sanchez V., Chakrabarty A., Dave B., Cook R.S., Pao W., McKinely E., et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl. Acad. Sci. USA. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 174. Chen C.-T., Kim H., Liska D., Gao S., Christensen J.G., Weiser M.R. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol. Cancer Ther. 2012;11:660–669. doi: 10.1158/1535-7163.MCT-11-0754. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 175. Trowe T., Boukouvala S., Calkins K., Cutler R.E., Fong R., Funke R., Gendreau S.B., Kim Y.D., Miller N., Woolfrey J.R., et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin. Cancer Res. 2008;14:2465–2475. doi: 10.1158/1078-0432.CCR-07-4367. [ DOI ] [ PubMed ] [ Google Scholar ]
- 176. Wetterskog D., Shiu K.-K., Chong I., Meijer T., Mackay A., Lambros M., Cunningham D., Reis-Filho J.S., Lord C.J., Ashworth A. Identification of novel determinants of resistance to lapatinib in ERBB2-amplified cancers. Oncogene. 2014;33:966–976. doi: 10.1038/onc.2013.41. [ DOI ] [ PubMed ] [ Google Scholar ]
- 177. Lewis Phillips G.D., Li G., Dugger D.L., Crocker L.M., Parsons K.L., Mai E., Blättler W.A., Lambert J.M., Chari R.V.J., Lutz R.J., et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [ DOI ] [ PubMed ] [ Google Scholar ]
- 178. Erickson H.K., Park P.U., Widdison W.C., Kovtun Y.V., Garrett L.M., Hoffman K., Lutz R.J., Goldmacher V.S., Blättler W.A. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66:4426–4433. doi: 10.1158/0008-5472.CAN-05-4489. [ DOI ] [ PubMed ] [ Google Scholar ]
- 179. Chari R.V.J. Targeted cancer therapy: Conferring specificity to cytotoxic drugs. Acc. Chem Res. 2008;41:98–107. doi: 10.1021/ar700108g. [ DOI ] [ PubMed ] [ Google Scholar ]
- 180. Junttila T.T., Li G., Parsons K., Phillips G.L., Sliwkowski M.X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 2011;128:347–356. doi: 10.1007/s10549-010-1090-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 181. Welslau M., Diéras V., Sohn J.-H., Hurvitz S.A., Lalla D., Fang L., Althaus B., Guardino E., Miles D. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014;120:642–651. doi: 10.1002/cncr.28465. [ DOI ] [ PubMed ] [ Google Scholar ]
- 182. Krop I.E., Kim S.-B., Martin A.G., LoRusso P.M., Ferrero J.-M., Badovinac-Crnjevic T., Hoersch S., Smitt M., Wildiers H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18:743–754. doi: 10.1016/S1470-2045(17)30313-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 183. Perez E.A., Barrios C., Eiermann W., Toi M., Im Y.-H., Conte P., Martin M., Pienkowski T., Pivot X., Burris H.A., et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2–Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. JCO. 2017;35:141–148. doi: 10.1200/JCO.2016.67.4887. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 184. Kovtun Y.V., Goldmacher V.S. Cell killing by antibody-drug conjugates. Cancer Lett. 2007;255:232–240. doi: 10.1016/j.canlet.2007.04.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 185. Hunter F.W., Barker H.R., Lipert B., Rothé F., Gebhart G., Piccart-Gebhart M.J., Sotiriou C., Jamieson S.M.F. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer. 2020;122:603–612. doi: 10.1038/s41416-019-0635-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 186. Scaltriti M., Rojo F., Ocana A., Anido J., Guzman M., Cortes J., Di Cosimo S., Matias-Guiu X., Ramon y Cajal S., Arribas J., et al. Expression of p95HER2, a Truncated Form of the HER2 Receptor, and Response to Anti-HER2 Therapies in Breast Cancer. J. Natl. Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [ DOI ] [ PubMed ] [ Google Scholar ]
- 187. Austin C.D., De Mazière A.M., Pisacane P.I., van Dijk S.M., Eigenbrot C., Sliwkowski M.X., Klumperman J., Scheller R.H. Endocytosis and Sorting of ErbB2 and the Site of Action of Cancer Therapeutics Trastuzumab and Geldanamycin. Mol. Biol. Cell. 2004;15:5268–5282. doi: 10.1091/mbc.e04-07-0591. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 188. Lee G.-H., Kim D.-S., Kim H.-T., Lee J.-W., Chung C.-H., Ahn T., Lim J.M., Kim I.-K., Chae H.-J., Kim H.-R. Enhanced Lysosomal Activity Is Involved in Bax Inhibitor-1-induced Regulation of the Endoplasmic Reticulum (ER) Stress Response and Cell Death against ER Stress. J. Biol. Chem. 2011;286:24743–24753. doi: 10.1074/jbc.M110.167734. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 189. Liddil J.D., Dorr R.T., Scuderi P. Association of lysosomal activity with sensitivity and resistance to tumor necrosis factor in murine L929 cells. Cancer Res. 1989;49:2722–2728. [ PubMed ] [ Google Scholar ]
- 190. Barok M., Joensuu H., Isola J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 191. Gavet O., Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell. 2010;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 192. Wang J., Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Sig. Transduct. Target. Ther. 2019;4:34. doi: 10.1038/s41392-019-0069-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 193. Chen H., Wu J., Zhang Z., Tang Y., Li X., Liu S., Cao S., Li X. Association Between BRCA Status and Triple-Negative Breast Cancer: A Meta-Analysis. Front. Pharmacol. 2018;9:909. doi: 10.3389/fphar.2018.00909. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 194. Li M., Yu X. Function of BRCA1 in the DNA Damage Response Is Mediated by ADP-Ribosylation. Cancer Cell. 2013;23:693–704. doi: 10.1016/j.ccr.2013.03.025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 195. Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.-A., Mooij T.M., Roos-Blom M.-J., Jervis S., van Leeuwen F.E., Milne R.L., Andrieu N., et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [ DOI ] [ PubMed ] [ Google Scholar ]
- 196. Golia B., Singh H.R., Timinszky G. Poly-ADP-ribosylation signaling during DNA damage repair. Front. Biosci. 2015;20:440–457. doi: 10.2741/4318. [ DOI ] [ PubMed ] [ Google Scholar ]
- 197. Farmer H., McCabe N., Lord C.J., Tutt A.N.J., Johnson D.A., Richardson T.B., Santarosa M., Dillon K.J., Hickson I., Knights C., et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [ DOI ] [ PubMed ] [ Google Scholar ]
- 198. Zimmer A.S., Gillard M., Lipkowitz S., Lee J.-M. Update on PARP Inhibitors in Breast Cancer. Curr. Treat. Options Oncol. 2018;19:21. doi: 10.1007/s11864-018-0540-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 199. Griguolo G., Dieci M.V., Guarneri V., Conte P. Olaparib for the treatment of breast cancer. Expert Rev. Anticancer Ther. 2018;18:519–530. doi: 10.1080/14737140.2018.1458613. [ DOI ] [ PubMed ] [ Google Scholar ]
- 200. Fong P.C., Boss D.S., Yap T.A., Tutt A., Wu P., Mergui-Roelvink M., Mortimer P., Swaisland H., Lau A., O’Connor M.J., et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [ DOI ] [ PubMed ] [ Google Scholar ]
- 201. Tutt A., Robson M., Garber J.E., Domchek S.M., Audeh M.W., Weitzel J.N., Friedlander M., Arun B., Loman N., Schmutzler R.K., et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 202. Gelmon K.A., Tischkowitz M., Mackay H., Swenerton K., Robidoux A., Tonkin K., Hirte H., Huntsman D., Clemons M., Gilks B., et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 203. Kaufman B., Shapira-Frommer R., Schmutzler R.K., Audeh M.W., Friedlander M., Balmaña J., Mitchell G., Fried G., Stemmer S.M., Hubert A., et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 204. Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [ DOI ] [ PubMed ] [ Google Scholar ]
- 205. Dent R.A., Lindeman G.J., Clemons M., Wildiers H., Chan A., McCarthy N.J., Singer C.F., Lowe E.S., Watkins C.L., Carmichael J. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013;15:R88. doi: 10.1186/bcr3484. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 206. Lee J.-M., Hays J.L., Annunziata C.M., Noonan A.M., Minasian L., Zujewski J.A., Yu M., Gordon N., Ji J., Sissung T.M., et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J. Natl. Cancer Inst. 2014;106:dju089. doi: 10.1093/jnci/dju089. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 207. Balmaña J., Tung N.M., Isakoff S.J., Graña B., Ryan P.D., Saura C., Lowe E.S., Frewer P., Winer E., Baselga J., et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann. Oncol. 2014;25:1656–1663. doi: 10.1093/annonc/mdu187. [ DOI ] [ PubMed ] [ Google Scholar ]
- 208. Murai J., Huang S.-Y.N., Renaud A., Zhang Y., Ji J., Takeda S., Morris J., Teicher B., Doroshow J.H., Pommier Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014;13:433–443. doi: 10.1158/1535-7163.MCT-13-0803. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 209. Litton J.K., Scoggins M., Ramirez D.L., Murthy R.K., Whitman G.J., Hess K.R., Adrada B.E., Moulder S.L., Barcenas C.H., Valero V., et al. A pilot study of neoadjuvant talazoparib for early-stage breast cancer patients with a BRCA mutation. Ann. Oncol. 2016;27:vi46. doi: 10.1093/annonc/mdw364.10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 210. Turner N.C., Telli M.L., Rugo H.S., Mailliez A., Ettl J., Grischke E.-M., Mina L.A., Balmana Gelpi J., Fasching P.A., Hurvitz S.A., et al. Final results of a phase 2 study of talazoparib (TALA) following platinum or multiple cytotoxic regimens in advanced breast cancer patients (pts) with germline BRCA1/2 mutations (ABRAZO) JCO. 2017;35:1007. doi: 10.1200/JCO.2017.35.15_suppl.1007. [ DOI ] [ Google Scholar ]
- 211. Loibl S., O’Shaughnessy J., Untch M., Sikov W.M., Rugo H.S., McKee M.D., Huober J., Golshan M., von Minckwitz G., Maag D., et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 212. Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., Fehrenbacher L., Yerushalmi R., Mina L.A., Martin M., et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 213. Rugo H.S., Olopade O.I., DeMichele A., Yau C., van’t Veer L.J., Buxton M.B., Hogarth M., Hylton N.M., Paoloni M., Perlmutter J., et al. Adaptive Randomization of Veliparib–Carboplatin Treatment in Breast Cancer. N. Engl. J. Med. 2016;375:23–34. doi: 10.1056/NEJMoa1513749. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 214. Han H.S., Diéras V., Robson M., Palácová M., Marcom P.K., Jager A., Bondarenko I., Citrin D., Campone M., Telli M.L., et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: Randomized phase II study. Ann. Oncol. 2018;29:154–161. doi: 10.1093/annonc/mdx505. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 215. Balasubramaniam S., Beaver J.A., Horton S., Fernandes L.L., Tang S., Horne H.N., Liu J., Liu C., Schrieber S.J., Yu J., et al. FDA Approval Summary: Rucaparib for the Treatment of Patients with Deleterious BRCA Mutation–Associated Advanced Ovarian Cancer. Clin. Cancer Res. 2017;23:7165–7170. doi: 10.1158/1078-0432.CCR-17-1337. [ DOI ] [ PubMed ] [ Google Scholar ]
- 216. Drew Y., Ledermann J., Hall G., Rea D., Glasspool R., Highley M., Jayson G., Sludden J., Murray J., Jamieson D., et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br. J. Cancer. 2016;114:723–730. doi: 10.1038/bjc.2016.41. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 217. Wilson R.H., Evans T.J., Middleton M.R., Molife L.R., Spicer J., Dieras V., Roxburgh P., Giordano H., Jaw-Tsai S., Goble S., et al. A phase I study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br. J. Cancer. 2017;116:884–892. doi: 10.1038/bjc.2017.36. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 218. Miller K., Tong Y., Jones D.R., Walsh T., Danso M.A., Ma C.X., Silverman P., King M.-C., Badve S.S., Perkins S.M. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple negative breast cancer: Final efficacy results of Hoosier Oncology Group BRE09-146. JCO. 2015;33:1082. doi: 10.1200/jco.2015.33.15_suppl.1082. [ DOI ] [ Google Scholar ]
- 219. D’Andrea A.D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair. 2018;71:172–176. doi: 10.1016/j.dnarep.2018.08.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 220. Sakai W., Swisher E.M., Karlan B.Y., Agarwal M.K., Higgins J., Friedman C., Villegas E., Jacquemont C., Farrugia D.J., Couch F.J., et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 221. Edwards S.L., Brough R., Lord C.J., Natrajan R., Vatcheva R., Levine D.A., Boyd J., Reis-Filho J.S., Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [ DOI ] [ PubMed ] [ Google Scholar ]
- 222. Dhillon K.K., Swisher E.M., Taniguchi T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011;102:663–669. doi: 10.1111/j.1349-7006.2010.01840.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 223. Drost R., Bouwman P., Rottenberg S., Boon U., Schut E., Klarenbeek S., Klijn C., van der Heijden I., van der Gulden H., Wientjens E., et al. BRCA1 RING Function Is Essential for Tumor Suppression but Dispensable for Therapy Resistance. Cancer Cell. 2011;20:797–809. doi: 10.1016/j.ccr.2011.11.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 224. Pettitt S.J., Krastev D.B., Brandsma I., Dréan A., Song F., Aleksandrov R., Harrell M.I., Menon M., Brough R., Campbell J., et al. Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat. Commun. 2018;9:1849. doi: 10.1038/s41467-018-03917-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 225. Ray Chaudhuri A., Callen E., Ding X., Gogola E., Duarte A.A., Lee J.-E., Wong N., Lafarga V., Calvo J.A., Panzarino N.J., et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature. 2016;535:382–387. doi: 10.1038/nature18325. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 226. Rondinelli B., Gogola E., Yücel H., Duarte A.A., van de Ven M., van der Sluijs R., Konstantinopoulos P.A., Jonkers J., Ceccaldi R., Rottenberg S., et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat. Cell Biol. 2017;19:1371–1378. doi: 10.1038/ncb3626. [ DOI ] [ PubMed ] [ Google Scholar ]
- 227. Lyons T.G. Targeted Therapies for Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2019;20:82. doi: 10.1007/s11864-019-0682-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 228. Bareche Y., Venet D., Ignatiadis M., Aftimos P., Piccart M., Rothe F., Sotiriou C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 2018;29:895–902. doi: 10.1093/annonc/mdy024. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 229. Brufsky A.M., Dickler M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist. 2018;23:528–539. doi: 10.1634/theoncologist.2017-0423. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 230. Baselga J., Im S.-A., Iwata H., Cortés J., De Laurentiis M., Jiang Z., Arteaga C.L., Jonat W., Clemons M., Ito Y., et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–916. doi: 10.1016/S1470-2045(17)30376-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 231. Di Leo A., Johnston S., Lee K.S., Ciruelos E., Lønning P.E., Janni W., O’Regan R., Mouret-Reynier M.-A., Kalev D., Egle D., et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 232. Martín M., Chan A., Dirix L., O’Shaughnessy J., Hegg R., Manikhas A., Shtivelband M., Krivorotko P., Batista López N., Campone M., et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4) Ann. Oncol. 2017;28:313–320. doi: 10.1093/annonc/mdw562. [ DOI ] [ PubMed ] [ Google Scholar ]
- 233. Krop I.E., Mayer I.A., Ganju V., Dickler M., Johnston S., Morales S., Yardley D.A., Melichar B., Forero-Torres A., Lee S.C., et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:811–821. doi: 10.1016/S1470-2045(16)00106-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 234. Vuylsteke P., Huizing M., Petrakova K., Roylance R., Laing R., Chan S., Abell F., Gendreau S., Rooney I., Apt D., et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann. Oncol. 2016;27:2059–2066. doi: 10.1093/annonc/mdw320. [ DOI ] [ PubMed ] [ Google Scholar ]
- 235. Mayer I.A., Abramson V.G., Formisano L., Balko J.M., Estrada M.V., Sanders M.E., Juric D., Solit D., Berger M.F., Won H.H., et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. 2017;23:26–34. doi: 10.1158/1078-0432.CCR-16-0134. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 236. André F., Ciruelos E., Rubovszky G., Campone M., Loibl S., Rugo H.S., Iwata H., Conte P., Mayer I.A., Kaufman B., et al. Alpelisib for PIK3CA -Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [ DOI ] [ PubMed ] [ Google Scholar ]
- 237. Mayer I.A., Prat A., Egle D., Blau S., Fidalgo J.A.P., Gnant M., Fasching P.A., Colleoni M., Wolff A.C., Winer E.P., et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB) Clin. Cancer Res. 2019;25:2975–2987. doi: 10.1158/1078-0432.CCR-18-3160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 238. Baselga J., Dent S.F., Cortés J., Im Y.-H., Diéras V., Harbeck N., Krop I.E., Verma S., Wilson T.R., Jin H., et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. JCO. 2018;36:LBA1006. doi: 10.1200/JCO.2018.36.18_suppl.LBA1006. [ DOI ] [ Google Scholar ]
- 239. Saura C., Hlauschek D., Oliveira M., Zardavas D., Jallitsch-Halper A., de la Peña L., Nuciforo P., Ballestrero A., Dubsky P., Lombard J.M., et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019;20:1226–1238. doi: 10.1016/S1470-2045(19)30334-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 240. Baselga J., Campone M., Piccart M., Burris H.A., Rugo H.S., Sahmoud T., Noguchi S., Gnant M., Pritchard K.I., Lebrun F., et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 241. Bachelot T., Bourgier C., Cropet C., Ray-Coquard I., Ferrero J.-M., Freyer G., Abadie-Lacourtoisie S., Eymard J.-C., Debled M., Spaëth D., et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J. Clin. Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [ DOI ] [ PubMed ] [ Google Scholar ]
- 242. Kornblum N., Zhao F., Manola J., Klein P., Ramaswamy B., Brufsky A., Stella P.J., Burnette B., Telli M., Makower D.F., et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. 2018;36:1556–1563. doi: 10.1200/JCO.2017.76.9331. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 243. Jones R.H., Casbard A., Carucci M., Cox C., Butler R., Alchami F., Madden T.-A., Bale C., Bezecny P., Joffe J., et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21:345–357. doi: 10.1016/S1470-2045(19)30817-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 244. Smyth L.M., Tamura K., Oliveira M., Ciruelos E.M., Mayer I.A., Sablin M.-P., Biganzoli L., Ambrose H.J., Ashton J., Barnicle A., et al. Capivasertib, an AKT Kinase Inhibitor, as Monotherapy or in Combination with Fulvestrant in Patients with AKT1E17K-Mutant, ER-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2020;26:3947–3957. doi: 10.1158/1078-0432.CCR-19-3953. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 245. Finn R.S., Martin M., Rugo H.S., Jones S., Im S.-A., Gelmon K., Harbeck N., Lipatov O.N., Walshe J.M., Moulder S., et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 246. Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.-A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 247. Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S., Campone M., Petrakova K., Blackwell K.L., Winer E.P., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018;29:1541–1547. doi: 10.1093/annonc/mdy155. [ DOI ] [ PubMed ] [ Google Scholar ]
- 248. Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.-A., Petrakova K., Bianchi G.V., Esteva F.J., Martín M., et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [ DOI ] [ PubMed ] [ Google Scholar ]
- 249. Sledge G.W., Toi M., Neven P., Sohn J., Inoue K., Pivot X., Burdaeva O., Okera M., Masuda N., Kaufman P.A., et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [ DOI ] [ PubMed ] [ Google Scholar ]
- 250. Goetz M.P., Toi M., Campone M., Sohn J., Paluch-Shimon S., Huober J., Park I.H., Trédan O., Chen S.-C., Manso L., et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [ DOI ] [ PubMed ] [ Google Scholar ]
- 251. Nunnery S.E., Mayer I.A. Targeting the PI3K/AKT/mTOR Pathway in Hormone-Positive Breast Cancer. Drugs. 2020;80:1685–1697. doi: 10.1007/s40265-020-01394-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 252. Akinleye A., Avvaru P., Furqan M., Song Y., Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 2013;6:88. doi: 10.1186/1756-8722-6-88. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 253. Utermark T., Rao T., Cheng H., Wang Q., Lee S.H., Wang Z.C., Iglehart J.D., Roberts T.M., Muller W.J., Zhao J.J. The p110 and p110 isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012;26:1573–1586. doi: 10.1101/gad.191973.112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 254. Ndubaku C.O., Heffron T.P., Staben S.T., Baumgardner M., Blaquiere N., Bradley E., Bull R., Do S., Dotson J., Dudley D., et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): A β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J. Med. Chem. 2013;56:4597–4610. doi: 10.1021/jm4003632. [ DOI ] [ PubMed ] [ Google Scholar ]
- 255. Wander S.A., Hennessy B.T., Slingerland J.M. Next-generation mTOR inhibitors in clinical oncology: How pathway complexity informs therapeutic strategy. J. Clin. Investig. 2011;121:1231–1241. doi: 10.1172/JCI44145. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 256. Kumar C.C., Madison V. AKT crystal structure and AKT-specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [ DOI ] [ PubMed ] [ Google Scholar ]
- 257. Carpten J.D., Faber A.L., Horn C., Donoho G.P., Briggs S.L., Robbins C.M., Hostetter G., Boguslawski S., Moses T.Y., Savage S., et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [ DOI ] [ PubMed ] [ Google Scholar ]
- 258. El Hachem G., Gombos A., Awada A. Recent advances in understanding breast cancer and emerging therapies with a focus on luminal and triple-negative breast cancer. F1000Research. 2019;8 doi: 10.12688/f1000research.17542.1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 259. Fry D.W., Harvey P.J., Keller P.R., Elliott W.L., Meade M., Trachet E., Albassam M., Zheng X., Leopold W.R., Pryer N.K., et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 2004;3:1427–1438. [ PubMed ] [ Google Scholar ]
- 260. Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.-S., Sonke G.S., Paluch-Shimon S., Campone M., Blackwell K.L., André F., Winer E.P., et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [ DOI ] [ PubMed ] [ Google Scholar ]
- 261. Rugo H.S., Rumble R.B., Macrae E., Barton D.L., Connolly H.K., Dickler M.N., Fallowfield L., Fowble B., Ingle J.N., Jahanzeb M., et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [ DOI ] [ PubMed ] [ Google Scholar ]
- 262. Gul A., Leyland-Jones B., Dey N., De P. A combination of the PI3K pathway inhibitor plus cell cycle pathway inhibitor to combat endocrine resistance in hormone receptor-positive breast cancer: A genomic algorithm-based treatment approach. Am. J. Cancer Res. 2018;8:2359–2376. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 263. Escrivá-de-Romaní S., Arumí M., Bellet M., Saura C. HER2-positive breast cancer: Current and new therapeutic strategies. Breast. 2018;39:80–88. doi: 10.1016/j.breast.2018.03.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 264. Modi S., Saura C., Yamashita T., Park Y.H., Kim S.-B., Tamura K., Andre F., Iwata H., Ito Y., Tsurutani J., et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 265. Saura C., Thistlethwaite F., Banerji U., Lord S., Moreno V., MacPherson I., Boni V., Rolfo C.D., de Vries E.G.E., Van Herpen C.M.L., et al. A phase I expansion cohorts study of SYD985 in heavily pretreated patients with HER2-positive or HER2-low metastatic breast cancer. JCO. 2018;36:1014. doi: 10.1200/JCO.2018.36.15_suppl.1014. [ DOI ] [ Google Scholar ]
- 266. Bang Y.J., Giaccone G., Im S.A., Oh D.Y., Bauer T.M., Nordstrom J.L., Li H., Chichili G.R., Moore P.A., Hong S., et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 2017;28:855–861. doi: 10.1093/annonc/mdx002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 267. Murthy R.K., Loi S., Okines A., Paplomata E., Hamilton E., Hurvitz S.A., Lin N.U., Borges V., Abramson V., Anders C., et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [ DOI ] [ PubMed ] [ Google Scholar ]
- 268. Park Y.H., Lee K.-H., Sohn J.H., Lee K.S., Jung K.H., Kim J.-H., Lee K.H., Ahn J.S., Kim T.-Y., Kim G.M., et al. A phase II trial of the pan-HER inhibitor poziotinib, in patients with HER2-positive metastatic breast cancer who had received at least two prior HER2-directed regimens: Results of the NOV120101-203 trial. Int. J. Cancer. 2018;143:3240–3247. doi: 10.1002/ijc.31651. [ DOI ] [ PubMed ] [ Google Scholar ]
- 269. Mittendorf E.A., Clifton G.T., Holmes J.P., Schneble E., van Echo D., Ponniah S., Peoples G.E. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014;25:1735–1742. doi: 10.1093/annonc/mdu211. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 270. Mittendorf E.A., Ardavanis A., Litton J.K., Shumway N.M., Hale D.F., Murray J.L., Perez S.A., Ponniah S., Baxevanis C.N., Papamichail M., et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016;7:66192–66201. doi: 10.18632/oncotarget.11751. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 271. Mittendorf E.A., Ardavanis A., Symanowski J., Murray J.L., Shumway N.M., Litton J.K., Hale D.F., Perez S.A., Anastasopoulou E.A., Pistamaltzian N.F., et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann. Oncol. 2016;27:1241–1248. doi: 10.1093/annonc/mdw150. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 272. Jhaveri K., Drago J.Z., Shah P.D., Wang R., Pareja F., Ratzon F., Iasonos A., Patil S., Rosen N., Fornier M.N., et al. A Phase I study of alpelisib in combination with trastuzumab and LJM716 in patients with PIK3CA-mutated HER2-positive metastatic breast cancer. Clin. Cancer Res. 2021 doi: 10.1158/1078-0432.CCR-21-0047. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 273. Jain S., Shah A.N., Santa-Maria C.A., Siziopikou K., Rademaker A., Helenowski I., Cristofanilli M., Gradishar W.J. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2018;171:371–381. doi: 10.1007/s10549-018-4792-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 274. Keegan N.M., Furney S.J., Walshe J.M., Gullo G., Kennedy M.J., Smith D., McCaffrey J., Kelly C.M., Egan K., Kerr J., et al. Phase Ib Trial of Copanlisib, A Phosphoinositide-3 Kinase (PI3K) Inhibitor, with Trastuzumab in Advanced Pre-Treated HER2-Positive Breast Cancer “PantHER”. Cancers. 2021;13:1225. doi: 10.3390/cancers13061225. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 275. Hurvitz S.A., Andre F., Jiang Z., Shao Z., Mano M.S., Neciosup S.P., Tseng L.-M., Zhang Q., Shen K., Liu D., et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. doi: 10.1016/S1470-2045(15)00051-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 276. André F., O’Regan R., Ozguroglu M., Toi M., Xu B., Jerusalem G., Masuda N., Wilks S., Arena F., Isaacs C., et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 277. Ciruelos E., Villagrasa P., Pascual T., Oliveira M., Pernas S., Paré L., Escrivá-de-Romaní S., Manso L., Adamo B., Martínez E., et al. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res. 2020;26:5820–5829. doi: 10.1158/1078-0432.CCR-20-0844. [ DOI ] [ PubMed ] [ Google Scholar ]
- 278. Goel S., Pernas S., Tan-Wasielewski Z., Barry W.T., Bardia A., Rees R., Andrews C., Tahara R.K., Trippa L., Mayer E.L., et al. Ribociclib Plus Trastuzumab in Advanced HER2-Positive Breast Cancer: Results of a Phase 1b/2 Trial. Clin. Breast Cancer. 2019;19:399–404. doi: 10.1016/j.clbc.2019.05.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 279. Tolaney S.M., Wardley A.M., Zambelli S., Hilton J.F., Troso-Sandoval T.A., Ricci F., Im S.-A., Kim S.-B., Johnston S.R., Chan A., et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): A randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:763–775. doi: 10.1016/S1470-2045(20)30112-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 280. Trail P.A., Dubowchik G.M., Lowinger T.B. Antibody drug conjugates for treatment of breast cancer: Novel targets and diverse approaches in ADC design. Pharmacol. Ther. 2018;181:126–142. doi: 10.1016/j.pharmthera.2017.07.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 281. Keam S.J. Trastuzumab Deruxtecan: First Approval. Drugs. 2020;80:501–508. doi: 10.1007/s40265-020-01281-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 282. Doi T., Shitara K., Naito Y., Shimomura A., Fujiwara Y., Yonemori K., Shimizu C., Shimoi T., Kuboki Y., Matsubara N., et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–1522. doi: 10.1016/S1470-2045(17)30604-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 283. Dokter W., Ubink R., van der Lee M., van der Vleuten M., van Achterberg T., Jacobs D., Loosveld E., van den Dobbelsteen D., Egging D., Mattaar E., et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: Introduction of a new duocarmycin-based linker-drug platform. Mol. Cancer Ther. 2014;13:2618–2629. doi: 10.1158/1535-7163.MCT-14-0040-T. [ DOI ] [ PubMed ] [ Google Scholar ]
- 284. Banerji U., van Herpen C.M.L., Saura C., Thistlethwaite F., Lord S., Moreno V., Macpherson I.R., Boni V., Rolfo C., de Vries E.G.E., et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–1135. doi: 10.1016/S1470-2045(19)30328-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 285. Tarantino P., Morganti S., Uliano J., Giugliano F., Crimini E., Curigliano G. Margetuximab for the treatment of HER2-positive metastatic breast cancer. Expert Opin. Biol. Ther. 2021;21:127–133. doi: 10.1080/14712598.2021.1856812. [ DOI ] [ PubMed ] [ Google Scholar ]
- 286. Rugo H.S., Im S.-A., Cardoso F., Cortés J., Curigliano G., Musolino A., Pegram M.D., Wright G.S., Saura C., Escrivá-de-Romaní S., et al. Efficacy of Margetuximab vs. Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 287. De Nardis C., Hendriks L.J.A., Poirier E., Arvinte T., Gros P., Bakker A.B.H., de Kruif J. A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. J. Biol. Chem. 2017;292:14706–14717. doi: 10.1074/jbc.M117.793497. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 288. Geuijen C.A.W., De Nardis C., Maussang D., Rovers E., Gallenne T., Hendriks L.J.A., Visser T., Nijhuis R., Logtenberg T., de Kruif J., et al. Unbiased Combinatorial Screening Identifies a Bispecific IgG1 that Potently Inhibits HER3 Signaling via HER2-Guided Ligand Blockade. Cancer Cell. 2018;33:922–936.e10. doi: 10.1016/j.ccell.2018.04.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 289. Bartsch R., Bergen E. ASCO 2018: Highlights in HER2-positive metastatic breast cancer. Memo-Mag. Eur. Med. Oncol. 2018;11:280–283. doi: 10.1007/s12254-018-0441-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 290. Trabolsi A., Arumov A., Schatz J.H. T Cell–Activating Bispecific Antibodies in Cancer Therapy. J. Immunol. 2019;203:585–592. doi: 10.4049/jimmunol.1900496. [ DOI ] [ PubMed ] [ Google Scholar ]
- 291. Marmé F. Immunotherapy in Breast Cancer. Oncol. Res. Treat. 2016;39:335–345. doi: 10.1159/000446340. [ DOI ] [ PubMed ] [ Google Scholar ]
- 292. Arab A., Yazdian-Robati R., Behravan J. HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development. Arch. Immunol Ther. Exp. 2020;68:2. doi: 10.1007/s00005-019-00566-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 293. Datta J., Xu S., Rosemblit C., Smith J.B., Cintolo J.A., Powell D.J., Czerniecki B.J. CD4(+) T-Helper Type 1 Cytokines and Trastuzumab Facilitate CD8(+) T-cell Targeting of HER2/neu-Expressing Cancers. Cancer Immunol. Res. 2015;3:455–463. doi: 10.1158/2326-6066.CIR-14-0208. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 294. Clive K.S., Tyler J.A., Clifton G.T., Holmes J.P., Ponniah S., Peoples G.E., Mittendorf E.A. The GP2 peptide: A HER2/neu-based breast cancer vaccine. J. Surg. Oncol. 2012;105:452–458. doi: 10.1002/jso.21723. [ DOI ] [ PubMed ] [ Google Scholar ]
- 295. Carmichael M.G., Benavides L.C., Holmes J.P., Gates J.D., Mittendorf E.A., Ponniah S., Peoples G.E. Results of the first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine in disease-free breast cancer patients: United States Military Cancer Institute Clinical Trials Group Study I-04. Cancer. 2010;116:292–301. doi: 10.1002/cncr.24756. [ DOI ] [ PubMed ] [ Google Scholar ]
- 296. Gupta S. Intention-to-treat concept: A review. Perspect Clin. Res. 2011;2:109. doi: 10.4103/2229-3485.83221. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 297. Conlon N.T., Kooijman J.J., van Gerwen S.J.C., Mulder W.R., Zaman G.J.R., Diala I., Eli L.D., Lalani A.S., Crown J., Collins D.M. Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors. Br. J. Cancer. 2021;124:1249–1259. doi: 10.1038/s41416-020-01257-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 298. Metzger Filho O., Leone J.P., Li T., Tan-Wasielewski Z., Trippa L., Barry W.T., Younger J., Lawler E., Walker L., Freedman R.A., et al. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann. Oncol. 2020;31:1231–1239. doi: 10.1016/j.annonc.2020.05.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 299. Borges V.F., Ferrario C., Aucoin N., Falkson C., Khan Q., Krop I., Welch S., Conlin A., Chaves J., Bedard P.L., et al. Tucatinib Combined With Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2018;4:1214–1220. doi: 10.1001/jamaoncol.2018.1812. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 300. Shah M., Wedam S., Cheng J., Fiero M.H., Xia H., Li F., Fan J., Zhang X., Yu J., Song P., et al. FDA Approval Summary: Tucatinib for the Treatment of Patients with Advanced or Metastatic HER2-positive Breast Cancer. Clin. Cancer Res. 2021;27:1220–1226. doi: 10.1158/1078-0432.CCR-20-2701. [ DOI ] [ PubMed ] [ Google Scholar ]
- 301. Kim E., Kim H., Suh K., Kwon S., Lee G., Park N.H., Hong J. Metabolite identification of a new tyrosine kinase inhibitor, HM781-36B, and a pharmacokinetic study by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2013;27:1183–1195. doi: 10.1002/rcm.6559. [ DOI ] [ PubMed ] [ Google Scholar ]
- 302. Kim T.M., Lee K.-W., Oh D.-Y., Lee J.-S., Im S.-A., Kim D.-W., Han S.-W., Kim Y.J., Kim T.-Y., Kim J.H., et al. Phase 1 Studies of Poziotinib, an Irreversible Pan-HER Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Cancer Res. Treat. 2018;50:835–842. doi: 10.4143/crt.2017.303. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 303. Vernieri C., Milano M., Brambilla M., Mennitto A., Maggi C., Cona M.S., Prisciandaro M., Fabbroni C., Celio L., Mariani G., et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: Current knowledge, new research directions and therapeutic perspectives. Crit. Rev. Oncol. Hematol. 2019;139:53–66. doi: 10.1016/j.critrevonc.2019.05.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 304. Liu N., Rowley B.R., Bull C.O., Schneider C., Haegebarth A., Schatz C.A., Fracasso P.R., Wilkie D.P., Hentemann M., Wilhelm S.M., et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [ DOI ] [ PubMed ] [ Google Scholar ]
- 305. André F., Hurvitz S., Fasolo A., Tseng L.-M., Jerusalem G., Wilks S., O’Regan R., Isaacs C., Toi M., Burris H., et al. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2-Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis From BOLERO-1 and BOLERO-3. J. Clin. Oncol. 2016;34:2115–2124. doi: 10.1200/JCO.2015.63.9161. [ DOI ] [ PubMed ] [ Google Scholar ]
- 306. Haley B., Batra K., Sahoo S., Froehlich T., Klemow D., Unni N., Ahn C., Rodriguez M., Hullings M., Frankel A.E. A Phase I/Ib Trial of PD 0332991 (Palbociclib) and T-DM1 in HER2-Positive Advanced Breast Cancer After Trastuzumab and Taxane Therapy. Clin. Breast Cancer. 2021 doi: 10.1016/j.clbc.2021.03.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 307. Pernas S., Tolaney S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019;11:1758835919833519. doi: 10.1177/1758835919833519. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 308. Bianchini G., Balko J.M., Mayer I.A., Sanders M.E., Gianni L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 309. Bardia A., Hurvitz S.A., Tolaney S.M., Loirat D., Punie K., Oliveira M., Brufsky A., Sardesai S.D., Kalinsky K., Zelnak A.B., et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [ DOI ] [ PubMed ] [ Google Scholar ]
- 310. Bell R., Brown J., Parmar M., Toi M., Suter T., Steger G.G., Pivot X., Mackey J., Jackisch C., Dent R., et al. Final efficacy and updated safety results of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab-containing therapy in triple-negative early breast cancer. Ann. Oncol. 2017;28:754–760. doi: 10.1093/annonc/mdw665. [ DOI ] [ PubMed ] [ Google Scholar ]
- 311. Sikov W.M., Berry D.A., Perou C.M., Singh B., Cirrincione C.T., Tolaney S.M., Kuzma C.S., Pluard T.J., Somlo G., Port E.R., et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J. Clin. Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 312. Carey L.A., Rugo H.S., Marcom P.K., Mayer E.L., Esteva F.J., Ma C.X., Liu M.C., Storniolo A.M., Rimawi M.F., Forero-Torres A., et al. TBCRC 001: Randomized Phase II Study of Cetuximab in Combination With Carboplatin in Stage IV Triple-Negative Breast Cancer. JCO. 2012;30:2615–2623. doi: 10.1200/JCO.2010.34.5579. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 313. Baselga J., Gómez P., Greil R., Braga S., Climent M.A., Wardley A.M., Kaufman B., Stemmer S.M., Pêgo A., Chan A., et al. Randomized Phase II Study of the Anti–Epidermal Growth Factor Receptor Monoclonal Antibody Cetuximab With Cisplatin Versus Cisplatin Alone in Patients With Metastatic Triple-Negative Breast Cancer. JCO. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 314. Jovanović B., Mayer I.A., Mayer E.L., Abramson V.G., Bardia A., Sanders M.E., Kuba M.G., Estrada M.V., Beeler J.S., Shaver T.M., et al. A Randomized Phase II Neoadjuvant Study of Cisplatin, Paclitaxel With or Without Everolimus in Patients with Stage II/III Triple-Negative Breast Cancer (TNBC): Responses and Long-term Outcome Correlated with Increased Frequency of DNA Damage Response Gene Mutations, TNBC Subtype, AR Status, and Ki67. Clin. Cancer Res. 2017;23:4035–4045. doi: 10.1158/1078-0432.CCR-16-3055. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 315. Kim S.-B., Dent R., Im S.-A., Espié M., Blau S., Tan A.R., Isakoff S.J., Oliveira M., Saura C., Wongchenko M.J., et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18:1360–1372. doi: 10.1016/S1470-2045(17)30450-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 316. Oliveira M., Saura C., Nuciforo P., Calvo I., Andersen J., Passos-Coelho J.L., Gil Gil M., Bermejo B., Patt D.A., Ciruelos E., et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann. Oncol. 2019;30:1289–1297. doi: 10.1093/annonc/mdz177. [ DOI ] [ PubMed ] [ Google Scholar ]
- 317. Schmid P., Abraham J., Chan S., Wheatley D., Brunt A.M., Nemsadze G., Baird R.D., Park Y.H., Hall P.S., Perren T., et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel As First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J. Clin. Oncol. 2020;38:423–433. doi: 10.1200/JCO.19.00368. [ DOI ] [ PubMed ] [ Google Scholar ]
- 318. Gucalp A., Tolaney S., Isakoff S.J., Ingle J.N., Liu M.C., Carey L.A., Blackwell K., Rugo H., Nabell L., Forero A., et al. Phase II Trial of Bicalutamide in Patients with Androgen Receptor–Positive, Estrogen Receptor–Negative Metastatic Breast Cancer. Clin. Cancer Res. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 319. Traina T.A., Miller K., Yardley D.A., Eakle J., Schwartzberg L.S., O’Shaughnessy J., Gradishar W., Schmid P., Winer E., Kelly C., et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018;36:884–890. doi: 10.1200/JCO.2016.71.3495. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 320. Bonnefoi H., Grellety T., Tredan O., Saghatchian M., Dalenc F., Mailliez A., L’Haridon T., Cottu P., Abadie-Lacourtoisie S., You B., et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1) Ann. Oncol. 2016;27:812–818. doi: 10.1093/annonc/mdw067. [ DOI ] [ PubMed ] [ Google Scholar ]
- 321. Schmid P., Rugo H.S., Adams S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Henschel V., Molinero L., Chui S.Y., et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 322. Mittendorf E.A., Zhang H., Barrios C.H., Saji S., Jung K.H., Hegg R., Koehler A., Sohn J., Iwata H., Telli M.L., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 323. Loibl S., Untch M., Burchardi N., Huober J., Sinn B.V., Blohmer J.-U., Grischke E.-M., Furlanetto J., Tesch H., Hanusch C., et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [ DOI ] [ PubMed ] [ Google Scholar ]
- 324. Bachelot T., Filleron T., Bieche I., Arnedos M., Campone M., Dalenc F., Coussy F., Sablin M.-P., Debled M., Lefeuvre-Plesse C., et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat. Med. 2021;27:250–255. doi: 10.1038/s41591-020-01189-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 325. Zimmer A.S., Nichols E., Cimino-Mathews A., Peer C., Cao L., Lee M.-J., Kohn E.C., Annunziata C.M., Lipkowitz S., Trepel J.B., et al. A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1-3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. J. Immunother. Cancer. 2019;7:197. doi: 10.1186/s40425-019-0680-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 326. Dirix L.Y., Takacs I., Jerusalem G., Nikolinakos P., Arkenau H.-T., Forero-Torres A., Boccia R., Lippman M.E., Somer R., Smakal M., et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018;167:671–686. doi: 10.1007/s10549-017-4537-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 327. Adams S., Schmid P., Rugo H.S., Winer E.P., Loirat D., Awada A., Cescon D.W., Iwata H., Campone M., Nanda R., et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:397–404. doi: 10.1093/annonc/mdy517. [ DOI ] [ PubMed ] [ Google Scholar ]
- 328. Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.-A., Yusof M.M., Gallardo C., Lipatov O., Barrios C.H., Holgado E., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 329. Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., Denkert C., Park Y.H., Hui R., Harbeck N., et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [ DOI ] [ PubMed ] [ Google Scholar ]
- 330. Jiang D.M., Fyles A., Nguyen L.T., Neel B.G., Sacher A., Rottapel R., Wang B.X., Ohashi P.S., Sridhar S.S. Phase I study of local radiation and tremelimumab in patients with inoperable locally recurrent or metastatic breast cancer. Oncotarget. 2019;10:2947–2958. doi: 10.18632/oncotarget.26893. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 331. Dent R., Trudeau M., Pritchard K.I., Hanna W.M., Kahn H.K., Sawka C.A., Lickley L.A., Rawlinson E., Sun P., Narod S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [ DOI ] [ PubMed ] [ Google Scholar ]
- 332. Takahashi R., Toh U., Iwakuma N., Takenaka M., Otsuka H., Furukawa M., Fujii T., Seki N., Kawahara A., Kage M., et al. Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients. Breast Cancer Res. 2014;16:R70. doi: 10.1186/bcr3685. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 333. Miles D., Roché H., Martin M., Perren T.J., Cameron D.A., Glaspy J., Dodwell D., Parker J., Mayordomo J., Tres A., et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–1100. doi: 10.1634/theoncologist.2010-0307. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 334. Sahota S., Vahdat L.T. Sacituzumab govitecan: An antibody-drug conjugate. Expert Opin. Biol. Ther. 2017;17:1027–1031. doi: 10.1080/14712598.2017.1331214. [ DOI ] [ PubMed ] [ Google Scholar ]
- 335. Ripani E., Sacchetti A., Corda D., Alberti S. Human Trop-2 is a tumor-associated calcium signal transducer. Int. J. Cancer. 1998;76:671–676. doi: 10.1002/(SICI)1097-0215(19980529)76:5<671::AID-IJC10>3.0.CO;2-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 336. Bardia A., Mayer I.A., Diamond J.R., Moroose R.L., Isakoff S.J., Starodub A.N., Shah N.C., O’Shaughnessy J., Kalinsky K., Guarino M., et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017;35:2141–2148. doi: 10.1200/JCO.2016.70.8297. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 337. Bardia A., Mayer I.A., Vahdat L.T., Tolaney S.M., Isakoff S.J., Diamond J.R., O’Shaughnessy J., Moroose R.L., Santin A.D., Abramson V.G., et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [ DOI ] [ PubMed ] [ Google Scholar ]
- 338. Sussman D., Smith L.M., Anderson M.E., Duniho S., Hunter J.H., Kostner H., Miyamoto J.B., Nesterova A., Westendorf L., Van Epps H.A., et al. SGN-LIV1A: A novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol. Cancer Ther. 2014;13:2991–3000. doi: 10.1158/1535-7163.MCT-13-0896. [ DOI ] [ PubMed ] [ Google Scholar ]
- 339. Taylor K.M., Morgan H.E., Johnson A., Hadley L.J., Nicholson R.I. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem. J. 2003;375:51–59. doi: 10.1042/bj20030478. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 340. Modi S., Pusztai L., Forero A., Mita M., Miller K., Weise A., Krop I., Burris H., Kalinsky K., Tsai M., et al. Abstract PD3-14: Phase 1 Study of the Antibody-Drug Conjugate SGN-LIV1A in Patients with Heavily Pretreated Triple-Negative Metastatic Breast Cancer. Poster Discussion Abstracts. American Association for Cancer Research; Philadelphia, PA, USA: 2018. [(accessed on 22 May 2021)]. pp. PD3–PD14. Available online: http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.SABCS17-PD3-14 . [ Google Scholar ]
- 341. Masuda H., Zhang D., Bartholomeusz C., Doihara H., Hortobagyi G.N., Ueno N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012;136:331–345. doi: 10.1007/s10549-012-2289-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 342. Ribatti D., Nico B., Ruggieri S., Tamma R., Simone G., Mangia A. Angiogenesis and Antiangiogenesis in Triple-Negative Breast cancer. Transl. Oncol. 2016;9:453–457. doi: 10.1016/j.tranon.2016.07.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 343. Lee J.S., Yost S.E., Blanchard S., Schmolze D., Yin H.H., Pillai R., Robinson K., Tang A., Martinez N., Portnow J., et al. Phase I clinical trial of the combination of eribulin and everolimus in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2019;21:119. doi: 10.1186/s13058-019-1202-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 344. Niemeier L.A., Dabbs D.J., Beriwal S., Striebel J.M., Bhargava R. Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 2010;23:205–212. doi: 10.1038/modpathol.2009.159. [ DOI ] [ PubMed ] [ Google Scholar ]
- 345. Gerratana L., Basile D., Buono G., De Placido S., Giuliano M., Minichillo S., Coinu A., Martorana F., De Santo I., Del Mastro L., et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat. Rev. 2018;68:102–110. doi: 10.1016/j.ctrv.2018.06.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 346. Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 347. Coussens L.M., Zitvogel L., Palucka A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 348. Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 349. Schütz F., Stefanovic S., Mayer L., von Au A., Domschke C., Sohn C. PD-1/PD-L1 Pathway in Breast Cancer. Oncol. Res. Treat. 2017;40:294–297. doi: 10.1159/000464353. [ DOI ] [ PubMed ] [ Google Scholar ]
- 350. Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 351. Emens L.A., Cruz C., Eder J.P., Braiteh F., Chung C., Tolaney S.M., Kuter I., Nanda R., Cassier P.A., Delord J.-P., et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 352. Nanda R., Chow L.Q.M., Dees E.C., Berger R., Gupta S., Geva R., Pusztai L., Pathiraja K., Aktan G., Cheng J.D., et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 353. Adams S., Loi S., Toppmeyer D., Cescon D.W., De Laurentiis M., Nanda R., Winer E.P., Mukai H., Tamura K., Armstrong A., et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:405–411. doi: 10.1093/annonc/mdy518. [ DOI ] [ PubMed ] [ Google Scholar ]
- 354. Winer E.P., Lipatov O., Im S.-A., Goncalves A., Muñoz-Couselo E., Lee K.S., Schmid P., Tamura K., Testa L., Witzel I., et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:499–511. doi: 10.1016/S1470-2045(20)30754-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 355. Ibrahim N.K., Murray J.L. Clinical Development of the STn-KLH Vaccine (Theratope®) Clin. Breast Cancer. 2003;3:S139–S143. doi: 10.3816/CBC.2003.s.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 356. Nooka A.K., Wang M.L., Yee A.J., Kaufman J.L., Bae J., Peterkin D., Richardson P.G., Raje N.S. Assessment of Safety and Immunogenicity of PVX-410 Vaccine With or Without Lenalidomide in Patients With Smoldering Multiple Myeloma: A Nonrandomized Clinical Trial. JAMA Oncol. 2018;4:e183267. doi: 10.1001/jamaoncol.2018.3267. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (2.0 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

COMMENTS
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide with more than 2 million new cases in 2020. Its incidence and death rates have increased over the last three decades due to the change in risk factor profiles, better cancer registration, and cancer detection.
The article presents a review of the literature on breast carcinoma - a disease affecting women in the world. Abstract. Breast cancer is the most-commonly diagnosed malignant tumor in women in the world, as well as the first cause of death from malignant tumors. The incidence of breast cancer is constantly increasing in all regions of the world.
This article presents a comprehensive review of the Breast Cancer literature examining epidemiology, diagnosis, pathology, ‘‘benign’’ breast disease, breast carcinoma in situ syndromes, staging, and
Breast cancer (BC) is the second most common cancer worldwide and the most commonly occurring malignancy in women. There is growing evidence that lifestyle factors, including diet, body weight and physical activity, may be associated with higher BC risk.
New approaches have recently emerged such as immunotherapy, conjugated antibodies, and targeting other metabolic pathways. This review summarizes current BC treatments and explores the new treatment strategies from a personalized therapy perspective and the resulting challenges.
Breast cancer is the most common cancer diagnosed in women, accounting for more than 1 in 10 new cancer diagnoses annually, and is the second most common cause of cancer death among women worldwide. The risk factors for breast cancer are well established, and risk reduction plays a vital role in reducing the incidence of breast cancer.