An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The rough guide to systematic reviews and meta-analyses
G biondi-zoccai, m lotrionte.
- Author information
- Copyright and License information
Giuseppe Biondi-Zoccai, MD Division of Cardiology, University of Modena and Reggio Emilia, Via Del Pozzo, 71 - 41124 Modena, Italy; E-mail: [email protected]
Corresponding author.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License 3.0, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/3.0/ and http://creativecommons.org/licenses/by-nc/3.0/legalcode .
The hierarchy of evidence based medicine postulates that systematic reviews of homogenous randomized trials represent one of the uppermost levels of clinical evidence. Indeed, the current overwhelming role of systematic reviews, meta-analyses and meta-regression analyses in evidence based heath care calls for a thorough knowledge of the pros and cons of these study designs, even for the busy clinician. Despite this sore need, few succinct but thorough resources are available to guide users or would-be authors of systematic reviews. This article provides a rough guide to reading and, summarily, designing and conducting systematic reviews and meta-analyses
Keywords: meta-analysis, meta-regression, systematic review
Introduction
"I like to think of the meta-analytic process
as similar to being in a helicopter.
On the ground individual trees
are visible with high resolution.
This resolution diminishes as the helicopter
rises, and its place we begin
to see patterns not visible from the ground"
Ingram Olkin
Systematic reviews and meta-analyses are being used more extensively by researchers and practitioners, thanks to the appeal of a single piece of literature that is immediately able to summarize diverse data on a specific topic [ 1 , 2 ]. They have been established as the most quoted and read article types, even toppling randomized clinical trials, and thus they are likely to play a progressively even greater role in the future of medicine [ 3 , 4 ]. In addition, they are often published in the most prestigious international peer-reviewed journals, reaching thousands of physicians and researchers worldwide.
As with any other analytical and research tool with a long-standing history ( Table 1 ), systematic reviews and meta-analyses, despite their major strengths, are well known for several potential major weaknesses.
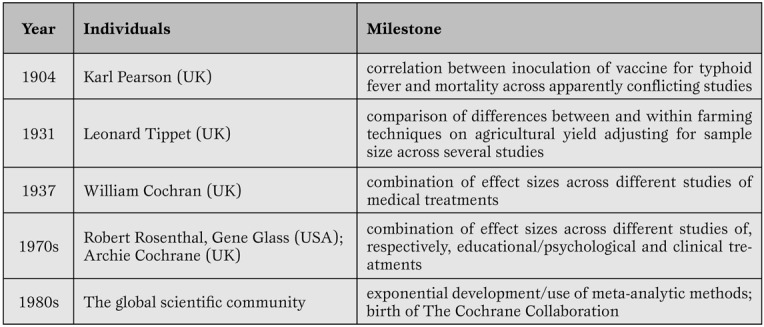
Key milestones in systematic review and meta-analysis development.
The aim of this review is to provide a concise but sound framework for the critical reading of systematic reviews and meta-analyses and, summarily, their design and conduct, stemming from our extensive experience with this type of research method ( Figure 1 ).
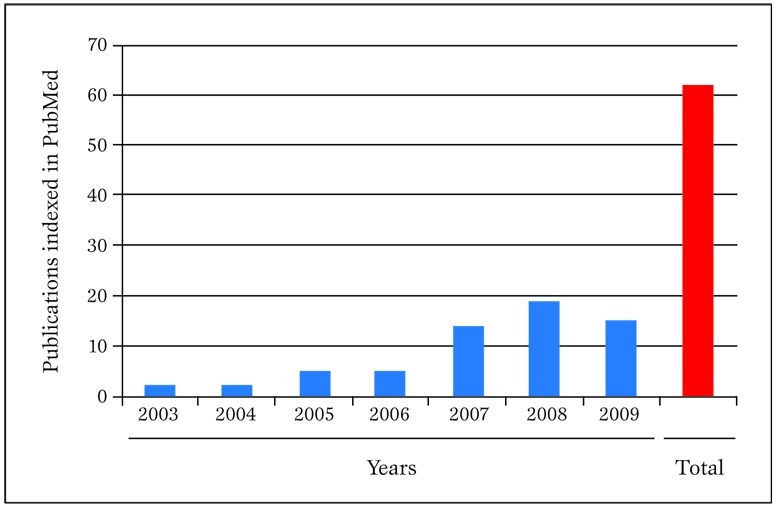
Publications in PubMed authored in the last few years by our research group concerning meta-analytic topics. Pubmed was searched on 30 March 2010 with the following strategy: "(biondi-zoccai OR Zoccai) AND (meta-analys* OR metaanalys* OR metaregress* OR "meta-regression")".
Definitions
A systematic review is a viewpoint focusing on a specific clinical problem, being it therapeutic, diagnostic or prognostic ( Table 2 ) [ 1 , 5 ].
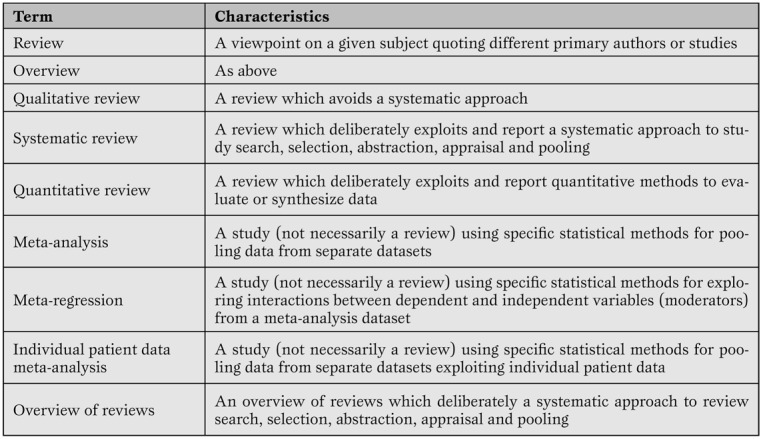
Minimal glossary pertinent to systematic reviews and meta-analyses.
The term systematic means that all the steps underlying the reviewing process are explicitly and clearly defined, and may be reproduced independently by other researchers. Thus, a formal set of methods is applied to study search (i.e. to the extensive search of primary/original studies), study selection, study appraisal, data abstraction and, when appropriate, data pooling according to statistical methods. Indeed, the term meta-analysis refers to a statistical method used to combine results from several different primary studies in order to provide more precise and valid results. Thus, not all systematic reviews include a meta-analysis, as not all topics are suitable for sound and robust data pooling. At the same time, meta-analysis can be conducted outside the realm of a systematic review (e.g. in the absence of extensive and thorough literature searches), but in such cases results of the meta-analytic efforts should be best viewed as hypothesis-generating only. This depends mainly on the fact that meta-analysis outside the framework of a systematic review has a major risk of publication bias.
Systematic reviews (especially when including meta-analytic pooling of quantitative data) have several unique strengths [ 1 , 5 ]. Specifically, they exploit systematic literature searches enabling the retrieval of the whole body of evidence pertaining to a specific clinical question.
Their standardized methods for search, evaluation and selection of primary studies enable reproducibility and an objective stance. Individual primary studies undergo a thorough evaluation for internal validity, together with the identification of the risk for bias All too often, systematic reviews hold their greater strength precisely in their ability to pinpoint weaknesses and fallacies in apparently sound primary studies [ 6 ].
Quantitative synthesis by means of meta-analysis also substantially increases statistical power, and yields narrower confidence intervals for statistical inference. The assessment of the effect of an intervention (exposure or diagnostic test) across different settings and times provides estimates and inferences with much greater external validity. The larger sample sizes often achieved by systematic reviews may even offer ample room for testing post-hoc hypotheses or exploring the effects in selected subgroups [ 7 ]. Clinical and statistical variability (i.e. heterogeneity and inconsistency) may be exploited by advanced statistical methods such as meta-regression, possibly offering the opportunity to test novel and hitherto unprecedented hypotheses [ 8 ]. Finally, meta-regression methods can be used to perform adjusted indirect comparisons or network meta-analyses [ 9 ].
Limitations
Drawbacks of systematic reviews and meta-analyses are also substantial, and should never be dismissed [ 1 ]. Since the first critique of being “an exercise in mega-silliness” and inappropriately "mixing apples and oranges" [ 10 ], there has been ongoing debate on the most correct approach to choose when meta-analytic pooling should be pursued (e.g. in case of statistical homogeneity and consistency) and when, conversely, the reviewer should refrain from meta-analysis (e.g. in case of severe statistical heterogeneity [as testified by p values <0.10 at χ 2 test] or significant statistical inconsistency [as testified by I 2 values>50%]) [ 11 ].
Whereas Canadian authors suggest that systematic reviews and meta-analyses from homogenous randomized controlled trials represent the apex of the evidence-based medicine pyramid (discounting for the role of n of 1 randomized trials) [ 12 ], others maintain that very large and simple randomized clinical trials offer several premium features, and should always be preferred, when available, to systematic reviews [ 13 ].
It is also all too common to retrieve only a few studies which focus on a given clinical topic, or otherwise studies may be found, but of such low quality, that including or even discussing them in the setting of a systematic review may appear misleading. Indeed, in such cases the meta-analysis itself can be considered misleading. Nonetheless, key insights may be gained in these cases by exploring sources of heterogeneity, stratified analyses, and meta-regressions. This drawback is strictly associated with the major threat to meta-analysis validity called the small study effect (also, albeit inappropriately, called small study bias or publication bias) [ 1 ]. Indeed, it is common to recognize, especially in large datasets, that small primary studies are more likely to be reported, published and quoted if their results are significant. Conversely, small non-significant studies often fail to reach publication or dissemination, and may thus be very easily missed, even after thorough literature searches. Combining results from these “biased” small studies with those of larger studies (which are usually published even if negative or non-significant) may inappropriately deviate summary effect estimates away from the true value. Unfortunately, despite the availability of several graphical and analytical tests [ 14 ] small study effects (which actually encompass publication bias) are potentially always present in a systematic review and should never be disregarded.
In addition, in the ongoing worldwide research effort, it is all too common for reviewers to focus only on English language studies, and thus unduly restricting their search and excluding potentially important works (e.g. from China or Japan). Another common critique is that systematic reviews and meta-analyses are not original research ( Figure 2 )
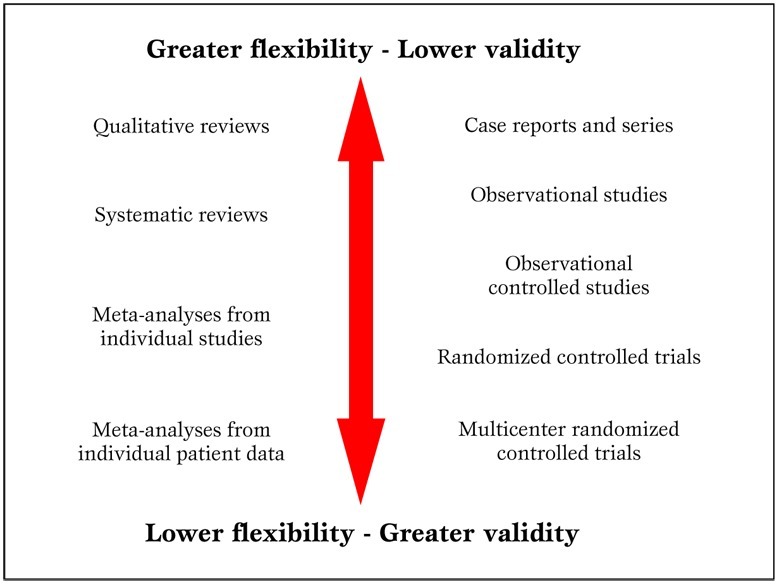
Parallel hierarchy of scientific studies in clinical research. Modified from Biondi-Zoccai et al. (2).
The reader is left free to form independently his informed opinion on this specific issue. Nonetheless, the main meter to judge a systematic review should be its novelty and usefulness for the very same reader, not whether it appears as original or secondary research [ 2 ].
Finally, a burning issue is whether results from large systematic reviews and meta-analyses can ever be applied to the single individual under our care. This question cannot be answered once and for all, and judgment should always be employed when considering the application of meta-analytic results to a specific patient. Unless proven otherwise by a significant test of interaction, all patients should be considered likely to similarly benefit from a specific treatment or diagnostic strategy [ 12 ].
Appraising primary studies, systematic reviews and meta-analyses
Unfortunately publication of a systematic review in a peer-reviewed journal is not definitive evidence of its internal validity and usefulness for the clinical practitioner or researcher [ 15 ]. Peer-review is not very accomplished in judging or improving the quality of scientific reviews, and many examples of bad or unsuccessful peer-reviewing efforts can be easily found. However, just as ”democracy is the worst form of government except all those other forms that have been tried” (Sir Winston Churchill), peer-review is the “worst” method used to evaluate scientific research except all other methods that have been tried so far. This applies to all clinical research products in general and so also applies to systematic reviews and meta-analyses. Thus, provided that meta-analyses are accurately and thoroughly reported, the burden of quality appraisal lies largely, as usual, in the eye of the beholder (i.e. the reader).
Assessment of primary research studies as well as systematic reviews and meta-analyses should be based on their internal validity and then, provided it is reasonably adequate, on their results and external validity [ 12 ]. Whereas interpretation of results and external validity of any research endeavor depends on the specific context of application, and is thus best left open to the individual judgment of the reader or decision-maker, internal validity can be evaluated in a rather structured and validated way. Recent guidance on the appraisal of the risk of bias in primary research studies within the context of a systematic review has been provided by The Cochrane Collaboration, and includes a separate assessment of the risk of selection, performance, attrition and adjudication bias ( Table 3 ) [ 16 ]
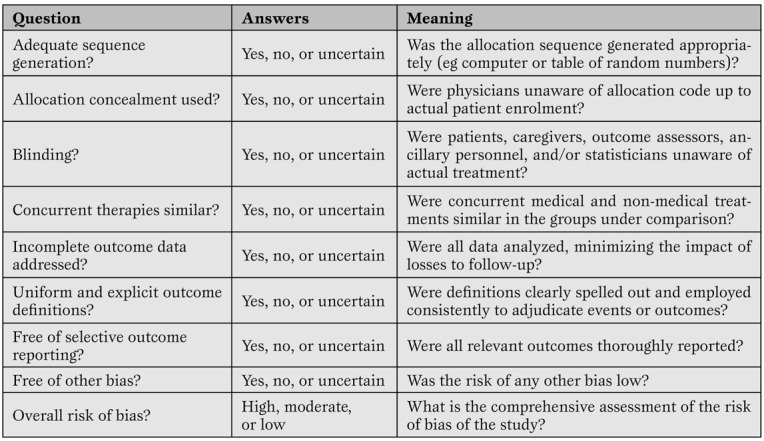
A modified version of The Cochrane Collaboration risk of bias assessment tool for the appraisal of primary studies.(16)*
Other valid and complementary approaches, targeted for specific study designs, have been proposed by advocates of evidence-based medicine methods, and include the Jadad score, the Delphi list, and the Megens-Harris list [ 12 ]. Nonetheless, even external validity can be formally evaluated by focusing on the population included, the control group, and result interpretation. Finally, established statistical criteria are available to determine whether a given intervention is effective and similar explicit criteria can inform on the presence of clinical significance.
The quality of a systematic review and meta-analysis depends on several factors, in particular the quality of the primary pooled studies.
Nonetheless, reporting quality (e.g. compliance with current guidelines on drafting and reporting of a meta-analysis by the Preferred Reporting Items for Systematic reviews and Meta-Analyses [PRISMA] or Meta-analysis Of Observational Studies in Epidemiology [MOOSE] statements) should be clearly distinguished by internal validity [ 17 , 18 ]. This can be low even in well reported reviews, whereas it is generally difficult to judge as highly valid a poorly reported systematic review and meta-analysis. The assessment of the internal validity of a review is quite complex and based on several assumptions, including study search and appraisal, methods for data pooling, and approaches to interpretation of study findings.
However, useful guidance was provided by Oxman and Guyatt with their well validated instrument ( Table 4 ) [ 19 ].

Oxman and Guyatt index for the appraisal of reviews. (19)*
More recently, other investigators have suggested other tools for the evaluation of systematic reviews, such as the A Measurement Tool to Assess Systematic Reviews (AMSTAR), and the Veritas plot, which await further validation ( Table 5 , Figure 3 ) [ 20 , 21 , 22 ].
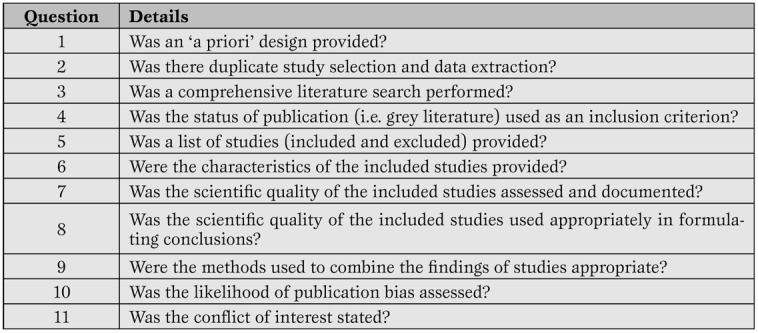
The AMSTAR tool for the appraisal of systematic reviews. (20-21)*
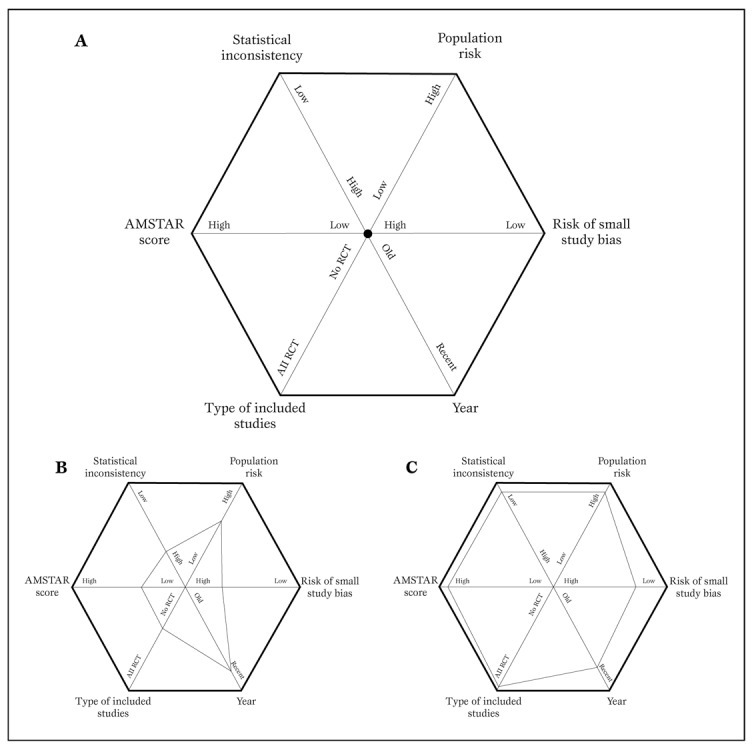
Typical diagram used to generate a Veritas plot (panel A) (22).
Using this tool, a low quality meta-analysis will be represented by a hexagon with a smaller area (panel B), whereas a high quality meta-analysis will be shown as a hexagon with a larger area (panel C).
For those busy critical care physicians wishing for a quicker approach to appraise systematic reviews, a simple two-step approach can be proposed. This is a simplification of the evidence-based medicine approach for the evaluation of sources of clinical evidence, but is nonetheless quite helpful [ 12 ]. Evidence-based medicine is “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients” [ 12 ]. It must also be stressed that “the practice of evidence-based medicine requires integration of individual clinical expertise and patient references with the best available external clinical evidence from systematic search” [ 12 ]. Systematic reviews and meta-analyses, if well conducted and reported, help us in reducing our efforts in looking for, evaluating, and summarizing the evidence.
But the burden of deciding what to do with the evidence obtained for the care of our individual patient remains ours.
Thus, the first step in appraising a systematic review and meta-analysis is to try and find an answer to the question: can I trust it? In other words, is this review internally valid, does it provide a precise and largely unbiased answer to its scientific question? Providing a definitive assessment of the internal validity of a systematic review is not a simple task, but largely depends on the methods employed and reported regarding study search, selection, abstraction, appraisal and, if appropriate, the study pooling. Even if we can conclude that a given meta-analysis is internally valid, we still have to face the second step in its evaluation. This focuses on the external validity of the study. In other words, can I apply the review results to the case I am facing or will shortly face? More basically it answers the question: so what? Decisions on external validity are highly subjective and may change depending on the clinical, historical, logistical, cultural or ethical context of the evaluator. Nonetheless, systematic reviews and meta-analyses can improve our appraisal of the external validity of any given clinical intervention, by suggesting an overall clinical efficacy (or lack of it).
It is clear that the assessment of the internal validity, and even more importantly the external validity, of any research endeavor, is highly subjective, and thus we leave ample room for the reader to enjoy and appraise them on his or her own.
The only issue that is worth being further stressed is that only collective and constructive, but critical post-publication evaluation of scientific studies can put and maintain them into the appropriate context for their correct and practical exploitation by the clinical researcher and the clinical practitioner.
Systematic reviews and meta-analyses: do it yourself
Even those not strictly committed to conduct a systematic review may obtain further insights into this clinical research method by understanding the key steps involved in the design, conduct and interpretation of a systematic review [ 5 ].
Briefly, a systematic review should always stem from a specific clinical question. Even if the experienced reviewer can probably informally guess the answer to this question the goal of the systematic review will be to confirm or disprove such hypothesis in a formal and structured way. With this goal in mind, the review should be designed as prospectively and in as much in detail as possible, to avoid conscious or unconscious manipulations of methods or data ( Figure 4 ).

Typical algorithm for the design and conduct of a systematic review. Modified from Biondi-Zoccai et al. (5).
The next steps are very important, and define the boundaries of the reviewing effort. Specifically, the reviewer should spell out the population of interest, the intervention or exposure to be appraised, the comparison(s) or comparator(s), and the outcome(s). The acronym PICO is often used to remember this approach. As an example, we could be interested in conducting a systematic review focusing on a population (P) of diabetics with coronary artery disease undergoing coronary artery bypass grafting, with the intervention (I) of interest being the administration of bivaridudin as anticoagulant, the comparator (C) being unfractioned heparin, and the outcomes (O) defined as in-hospital rates of death, myocardial infarction, stroke, or major bleeding (including bleeding needing repeat surgery).
After such preliminary steps, the actual review begins with a thorough and extensive search, encompassing several databases (not only MEDLINE/PubMed) with the help of library personnel experienced in literature searches, preferably also including conference abstracts and bibliographies of pertinent articles and reviews. When a list of potentially pertinent citations has been retrieved, these should be assessed and included/excluded based on criteria stemming directly from the PICO approach used to define the clinical question. Study appraisal also includes a formal evaluation of study validity and risk of bias of primary studies, whereas data abstraction, generally performed by at least two independent reviewers with divergences resolved after consensus, provides the quantitative data which will eventually be pooled with meta-analysis [ 16 ].
Indeed, provided that studies are relatively homogeneous and consistent, meta-analytic methods are employed to combine effect estimates from single studies into a unique summary effect estimate, with corresponding p values and confidence intervals for the effect ( Figure 5 )
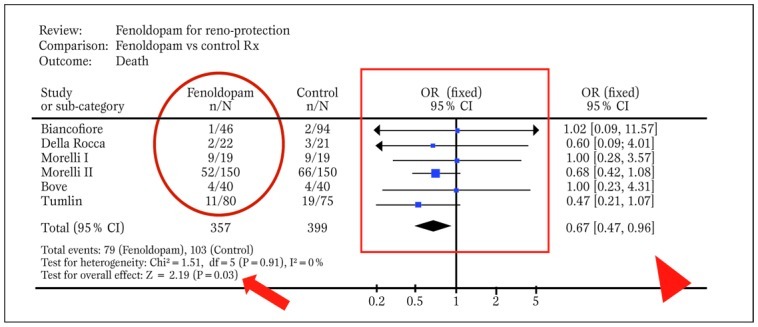
Typical forest plot generated by RevMan from a systematic review with meta-analytic pooling of dichotomous outcomes (df=degrees of freedom; E=expected cases; O=observed cases; OR=odds ratio). The solid oval highlights event counts in one of the groups under comparison, the solid box shows graphically individual and pooled point effect estimates with 95% confidence intervals, the arrowhead indicates the exact pooled point effect estimate with 95% confidence intervals (CI), the arrow shows the p value for effect, and the dashed oval highlights p value for statistical eterogeneity and measure of statistical inconsistency (I2). Modified from Landoni et al. (30).
In many cases results may lead reviewers to go back to the original research question and revise their working hypothesis. The last step relies on the interpretation and dissemination (possibly through publication in a peer-reviewed journal) of the results.
More advanced analytical issues
Unless extensively powered low event rates may often be found in primary research studies (e.g. with>1000 patients enrolled or with selective recruitment of very high-risk subjects). This may lead to null counts in one or more of the groups undergoing comparison in a controlled trial, generating severe computational hurdles. Indeed, most statistical methods used for meta-analytic pooling require that at least one event has occurred in each study group.
When this is not the case in one or more of the groups under comparison, bias may be introduced with the common practice of adding 0.25 or 0.50 to each group without events [ 23 ]. On top of this, when no event has occurred in any group, comparisons are more challenging and data from such an underpowered studies cannot be pooled with standard meta-analytic methods, as variance of the effect estimate approaches infinity. Nonetheless, other approaches (e.g. risk difference, continuity correction or Peto method) can still be used in case of total zero event trials.
Even when all groups undergoing comparison in a specific study have one or more events, the risk of biased estimates and alpha error (i.e. the risk of erroneously dismissing a null hypothesis despite it being true) may be present [ 1 ].
Indeed, minor differences in populations with few and rare events may provide nominally significant results (e.g. p=0.048) which however appear quite unstable. In such cases, we recommend reliance on the combined use of p values and 95% confidence intervals, or even making use of 99% confidence intervals. In other cases, a useful rule of thumb is to trust only meta-analyses reporting on at least 100 pooled events per group under comparison.
The risk of erroneously accepting a null hypothesis despite it being false (i.e. the beta error) is also common in systematic reviews and meta-analyses, especially when they include few studies with low event counts. This lack of statistical power (defined as 1-beta) is even more common with meta-regression analyses, which are usually underpowered because of few included studies and regression to the mean [ 7 ].
Surrogates may provide an important contribution to clinical research design, by increasing statistical power and offering insights in more than one clinical dimension. However, surrogate end-points (e.g. >25% increase in serum creatinine from baseline values to identify subclinical renal injury) may be less clinically relevant than hard clinical end-points (death or permanent need for hemodialysis) [ 12 ].
Usually, only surrogates which have a direct impact on patient well being and are independently associated with hard clinical end-points should be accepted for the design of clinical research studies. In any case, a study reaching significance based on surrogate end-points alone, but missing significance on analysis of hard end-points should be considered as hypothesis-generating or, at best, underpowered.
Small study bias always potentially threatens the results of a systematic review, as this type of confounding applies to all clinical topics and research study designs ( Figure 6 ) [ 24 ]
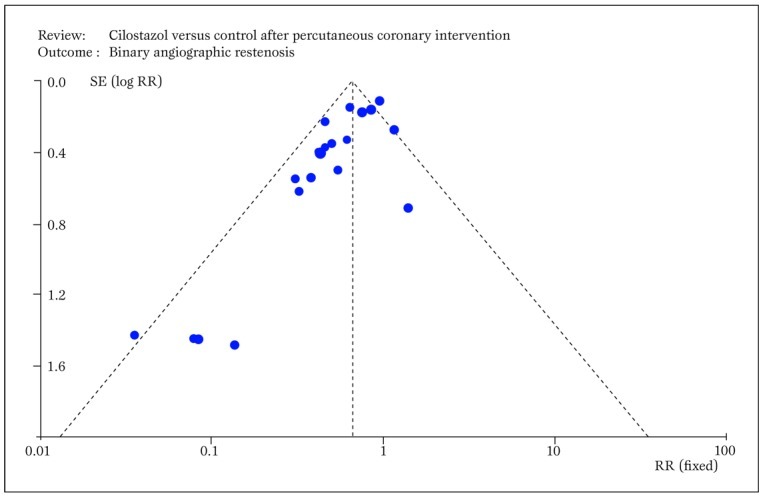
Typical funnel plot generated by RevMan showing small study bias, ie the asymmetric distribution of effect sizes in function of study precision, with selective publication of only positive small sample studies (RR=relative risk; SE=standard error). Modified from Biondi-Zoccai et al. (24).
Although this bias may be less significant in more recent and well financed drug or device studies (e.g. fenoldopam), in older or less well funded studies publication bias may profoundly undermine the results of a systematic review.
This has been all too evident in studies examining the role of acetylcysteine for the prevention of contrast-associated nephropathy [ 25 ], but is also obvious in other commonly prescribed agents. Another major threat to the validity of a systematic review, as to any other research endeavor, lies in conflicts of interest and study funding. It is well known that reviewers with underlying financial conflicts of interest are more likely to conclude in favor of the intervention benefiting the source of financial gains [ 26 ].
Whether these facts should lead to a more critical reading of their work or a comprehensive re-evaluation of their whole research project is best left at the readers’ discretion, but this should also take into account the overall internal validity (e.g. blinding of patients, physicians, adjudicators, and analysts) of the work.
Conclusions
Systematic reviews and meta-analyses offer powerful methods to evaluate the clinical effects of health interventions, especially when directly applied to real world clinical practice (such as in the Best Evidence Topic [BET] approach) [ 27 ].
More collaborative efforts are however required to design, conduct and disseminate individual patient data meta-analyses in an unbiased and rigorous manner [ 28 , 29 ].
Source of Support Nil.
Conflict of interest None declared.
Cite as: Biondi-Zoccai G, Lotrionte M, Landoni G, Modena MG. The rough guide to systematic reviews and meta-analyses. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2011; 3(3): 161-173
- Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. BMJ Publishing Group, London. 2001;2nd ed [ Google Scholar ]
- Biondi-Zoccai GG, Agostoni P, Abbate A. Parallel hierarchy of scientific studies in cardiovascular medicine. Ital Heart J. 2003;4:819–820. [ PubMed ] [ Google Scholar ]
- Patsopoulos NA, Analatos AA, Ioannidis JP. Relative citation impact of various study designs in the health sciences. JAMA. 2005;293:2362–2366. doi: 10.1001/jama.293.19.2362. [ DOI ] [ PubMed ] [ Google Scholar ]
- Glasziou P, Djulbegovic B, Burls A. Are systematic reviews more cost-effective than randomised trials? Lancet. 2006;367:2057–2058. doi: 10.1016/S0140-6736(06)68919-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- Biondi-Zoccai GG, Testa L, Agostoni P. A practical algorithm for systematic reviews in cardiovascular medicine. Ital Heart J. 2004;5:486–487. [ PubMed ] [ Google Scholar ]
- Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet. 1998;351:123–127. doi: 10.1016/S0140-6736(97)08468-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- Thompson SG, Higgins JP. How should meta-regression analyses undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [ DOI ] [ PubMed ] [ Google Scholar ]
- Biondi-Zoccai GG, Abbate A, Agostoni P. et al. Long-term benefits of an early invasive management in acute coronary syndromes depend on intracoronary stenting and aggressive antiplatelet treatment: a metaregression. Am Heart J. 2005;149:504–511. doi: 10.1016/j.ahj.2004.10.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- Glass G. Primary, secondary and meta-analysis of research. Educ Res. 1976;5:3–8. [ Google Scholar ]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Guyatt G, Rennie D, Meade M, Cook D. Users\' guides to the medical literature. A manual for evidence-based clinical practice. AMA Press, Chicago. 2002 [ Google Scholar ]
- Cappelleri JC, Ioannidis JP, Schmid CH. et al. Large trials vs meta-analysis of smaller trials: how do their results compare? JAMA. 1996;276:1332–1338. [ PubMed ] [ Google Scholar ]
- Peters J L, Sutton AJ, Jones DR. et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [ DOI ] [ PubMed ] [ Google Scholar ]
- Opthof T, Coronel R, Janse MJ. The significance of the peer review process against the background of bias: priority ratings of reviewers and editors and the prediction of citation, the role of geographical bias. Cardiovasc Res. 2002;56:339–346. doi: 10.1016/s0008-6363(02)00712-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, Oxford. 2008 [ Google Scholar ]
- Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:2700. doi: 10.1136/bmj.b2700. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stroup DF, Berlin JA, Morton SC. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [ DOI ] [ PubMed ] [ Google Scholar ]
- Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991;44:1271–1278. doi: 10.1016/0895-4356(91)90160-b. [ DOI ] [ PubMed ] [ Google Scholar ]
- Shea BJ, Grimshaw JM, Wells GA. et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shea BJ, Bouter LM, Peterson J. et al. External validation of a measurement tool to assess systematic reviews (AMSTAR) PLoS One. 2007;2:1350. doi: 10.1371/journal.pone.0001350. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Panesar SS, Rao C, Vecht JA. et al. Development of the Veritas plot and its application in cardiac surgery: an evidence-synthesis graphic tool for the clinician to assess multiple meta-analyses reporting on a common outcome. Can J Surg. 2009;52:137–145. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Golder S, Loke Y, McIntosh H. Room for improvement? A survey of the methods used in systematic reviews of adverse effects. BMC Med Res Methodol. 2006;6:3. doi: 10.1186/1471-2288-6-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Biondi-Zoccai GG, Lotrionte M, Anselmino M. et al. Systematic review and meta-analysis of randomized clinical trials appraising the impact of cilostazol after percutaneous coronary intervention. Am Heart J. 2008;155:1081–1089. doi: 10.1016/j.ahj.2007.12.024. [ DOI ] [ PubMed ] [ Google Scholar ]
- Biondi-Zoccai GG, Lotrionte M, Abbate A. et al. Compliance with QUOROM and quality of reporting of overlapping meta-analyses on the role of acetylcysteine in the prevention of contrast associated nephropathy: case study. BMJ. 2006;332:202–209. doi: 10.1136/bmj.38693.516782.7C. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barnes DE, Bero LA. Why review articles on the health effects of passive smoking reach different conclusions. JAMA. 1998;297:1566–1570. doi: 10.1001/jama.279.19.1566. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dunning J, Prendergast B, Mackway-Jones K. Towards evidence-based medicine in cardiothoracic surgery: best BETS. Interact Cardiovasc Thorac Surg. 2003;2:405–409. doi: 10.1016/S1569-9293(03)00191-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- De Luca G, Gibson CM, Bellandi F. et al. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty (EGYPT) cooperation: an individual patient data meta-analysis. Heart. 2008;94:1548–1558. doi: 10.1136/hrt.2008.141648. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Burzotta F, De Vita M, Gu YL. et al. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193–2203. doi: 10.1093/eurheartj/ehp348. [ DOI ] [ PubMed ] [ Google Scholar ]
- Landoni G, Biondi-Zoccai GG, Tumlin JA. et al. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007;49:56–68. doi: 10.1053/j.ajkd.2006.10.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- PDF (1.9 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

- Bodleian Libraries
- Oxford LibGuides
- Systematic Reviews and Evidence Syntheses
- Planning a Review
Systematic Reviews and Evidence Syntheses: Planning a Review
- Help and training
- Searching for studies
Planning your review
The planning stage of a systematic review is essential in avoiding the 5 most common mistakes in conducting systematic reviews and increasing the likelihood of future publication. The steps outlined on this page are often conducted concurrently.
- 5 most common mistakes in conducting systematic reviews
Formulating a question
- A well-built clinical question
A well-built clinical question is the cornerstone of evidence-based health care and has been incorporated into the production of systematic reviews. Formulating a question is the first and most essential step in preparing a protocol and will inform the whole review process, including:
- Choosing the most appropriate review method
- Providing the focus for the scoping searches
- Identifying the key concepts that will structure the search strategy
- Establishing the inclusion / exclusion criteria for study selection
- Structuring the data extraction and analysis methods
A question formulation tool or framework can be useful in breaking down a topic area into its key components. There are different tools for different types of review
- PICO = Population, Intervention, Comparison, Outcome
- PECO = Population, Exposure, Comparison, Outcome
- PIRT = Population, Index Test, Reference Test, Target Condition
- PCC = Population, Concept, Context
- SPiDER = Sample, Phenomenon of Interest, Design, Evaluation, Research Type
You should choose the most appropriate tool for your type of question. If your topic isn’t easily structured using one of these tools, you can remove elements or adapt the headings to make it work for your question. The key aspect of formulating a question is that you can take a topic and separate it into its component parts. Example Questions Here are 2 examples of how we might break down 2 different questions on the same topic of delayed antibiotic prescription: 1. How do delayed antibiotic prescriptions for respiratory infections affect patient & service outcomes compared to immediate /no prescription? Population = Patients with respiratory infections Intervention = Delayed antibiotic prescription Comparison = Immediate or no prescription Outcomes = time to recovery, repeat GP appointment, emergency hospitalisation, patient satisfaction... 2. What are the barriers and facilitators to implementing delayed antibiotic prescription to patients with respiratory infections attending primary care? Population = Patients with respiratory infections Concept = Barriers / Facilitators to implementing delayed antibiotic prescription Context = Primary Care
Scoping searches
Once you have defined your question you can start the process by conducting scoping searches. These are often simple searches for the key elements of your question. Using the example question above, we might search for:
Delayed prescribing AND antibiotics AND respiratory tract infections
Step 1: Locating existing systematic reviews
Identifying existing reviews or review protocols has three main purposes:
To verify that your question hasn't already been answered – published review
To ascertain whether researchers are in the process of conducting a review on the same question – review protocol
To identify related systematic reviews that must be accessed so you can review the reference lists for identifying relevant primary studies.
Useful databases for identifying systematic reviews in health care
- Cochrane Database of Systematic Reviews (CDSR)
- Epistemonikos
- Pubmed Search PubMed and filter the results for Article Type – Systematic Review
- PROSPERO: International prospective register of systematic reviews - the protocols of systematic reviews in health care are often registered on PROSPERO. For further suggestions on where to search, look at the next section ‘Developing and registering your protocol’
Step 2: Locating key studies
If you have existing knowledge of the topic area, you will already be aware of key studies that would provide background information for the review or be included in your final analysis. If not, you might want to conduct initial searches of a key database for your subject area (e.g. PubMed/Medline or Embase for healthcare) to inform the development of your protocol and search strategies. This will help with:
Refining the scope of your review – if you have too many or too few results on your initial searches, you might want to look again at your question
Informing the development of a search strategy – by reading relevant articles you’ll develop an understanding of the variability in terminology which will need to be incorporated into your final search
Writing the contextual / background information for your protocol
Developing and registering your protocol
Once you've formulated your question and established the need for a new review on your topic, you will need to start developing a protocol to guide the conduct of your review. This will cover inclusion/exclusion criteria, screening methods, risk of bias assessment and data analysis.
PRISMA-P provides guidance for the information that needs to be reported in a protocol for a systematic review. Systematic review protocol templates are available from PROSPERO . Scoping review templates are available from the Joanna Briggs Institute and OSF websites.
It is good practice to prospectively register your protocol and in many cases a requirement for future publication of the review. You may want to explore further why prospective registration of systematic reviews make sense .
Traditionally, PROSPERO has been the main repository for prospectively registering systematic review protocols in health care. In recent times, the development of alternative evidence reviews (e.g. scoping reviews) and the increase in preprint archives and collaborative open research platforms has increased the options available to researchers for prospective registration. In this case, you may need to further explore where to prospectively register a systematic review
Putting your team together
As the lead on a systematic review project, you will need to assemble a team of people around you that can provide methodological and topic support for your review. The size of the team around you will depend on the complexity of the question and potential volume of research that will need to be screened, quality assessed and synthesised. You will need people who can:
Develop, peer review and conduct searches
Double up on screening studies, extracting data and quality assessment
Provide methodological expertise in statistical, thematic or realist analysis
Supply topic / clinical expertise
Further Reading
- Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35(1):49-60.
- Gough, David (David A.), Sandy Oliver, and James Thomas, eds. An Introduction to Systematic Reviews. 2nd edition. Los Angeles ; SAGE, 2017. Print.
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023): Cochrane; 2023 [Available from: www.training.cochrane.org/handbook.
- << Previous: Home
- Next: Help and training >>
- Last Updated: Oct 24, 2024 2:58 PM
- URL: https://libguides.bodleian.ox.ac.uk/systematic-reviews
Website feedback
Accessibility Statement - https://visit.bodleian.ox.ac.uk/accessibility
Google Analytics - Bodleian Libraries use Google Analytics cookies on this web site. Google Analytics anonymously tracks individual visitor behaviour on this web site so that we can see how LibGuides is being used. We only use this information for monitoring and improving our websites and content for the benefit of our users (you). You can opt out of Google Analytics cookies completely (from all websites) by visiting https://tools.google.com/dlpage/gaoptout
© Bodleian Libraries 2021. Licensed under a Creative Commons Attribution 4.0 International Licence
Systematic Reviews and Meta Analysis
- Getting Started
- Guides and Standards
- Review Protocols
- Databases and Sources
- Randomized Controlled Trials
- Controlled Clinical Trials
- Observational Designs
- Tests of Diagnostic Accuracy
- Software and Tools
- Where do I get all those articles?
- Collaborations
- EPI 233/528
- Countway Mediated Search
- Risk of Bias (RoB)
Systematic review Q & A
What is a systematic review.
A systematic review is guided filtering and synthesis of all available evidence addressing a specific, focused research question, generally about a specific intervention or exposure. The use of standardized, systematic methods and pre-selected eligibility criteria reduce the risk of bias in identifying, selecting and analyzing relevant studies. A well-designed systematic review includes clear objectives, pre-selected criteria for identifying eligible studies, an explicit methodology, a thorough and reproducible search of the literature, an assessment of the validity or risk of bias of each included study, and a systematic synthesis, analysis and presentation of the findings of the included studies. A systematic review may include a meta-analysis.
For details about carrying out systematic reviews, see the Guides and Standards section of this guide.
Is my research topic appropriate for systematic review methods?
A systematic review is best deployed to test a specific hypothesis about a healthcare or public health intervention or exposure. By focusing on a single intervention or a few specific interventions for a particular condition, the investigator can ensure a manageable results set. Moreover, examining a single or small set of related interventions, exposures, or outcomes, will simplify the assessment of studies and the synthesis of the findings.
Systematic reviews are poor tools for hypothesis generation: for instance, to determine what interventions have been used to increase the awareness and acceptability of a vaccine or to investigate the ways that predictive analytics have been used in health care management. In the first case, we don't know what interventions to search for and so have to screen all the articles about awareness and acceptability. In the second, there is no agreed on set of methods that make up predictive analytics, and health care management is far too broad. The search will necessarily be incomplete, vague and very large all at the same time. In most cases, reviews without clearly and exactly specified populations, interventions, exposures, and outcomes will produce results sets that quickly outstrip the resources of a small team and offer no consistent way to assess and synthesize findings from the studies that are identified.
If not a systematic review, then what?
You might consider performing a scoping review . This framework allows iterative searching over a reduced number of data sources and no requirement to assess individual studies for risk of bias. The framework includes built-in mechanisms to adjust the analysis as the work progresses and more is learned about the topic. A scoping review won't help you limit the number of records you'll need to screen (broad questions lead to large results sets) but may give you means of dealing with a large set of results.
This tool can help you decide what kind of review is right for your question.
Can my student complete a systematic review during her summer project?
Probably not. Systematic reviews are a lot of work. Including creating the protocol, building and running a quality search, collecting all the papers, evaluating the studies that meet the inclusion criteria and extracting and analyzing the summary data, a well done review can require dozens to hundreds of hours of work that can span several months. Moreover, a systematic review requires subject expertise, statistical support and a librarian to help design and run the search. Be aware that librarians sometimes have queues for their search time. It may take several weeks to complete and run a search. Moreover, all guidelines for carrying out systematic reviews recommend that at least two subject experts screen the studies identified in the search. The first round of screening can consume 1 hour per screener for every 100-200 records. A systematic review is a labor-intensive team effort.
How can I know if my topic has been been reviewed already?
Before starting out on a systematic review, check to see if someone has done it already. In PubMed you can use the systematic review subset to limit to a broad group of papers that is enriched for systematic reviews. You can invoke the subset by selecting if from the Article Types filters to the left of your PubMed results, or you can append AND systematic[sb] to your search. For example:
"neoadjuvant chemotherapy" AND systematic[sb]
The systematic review subset is very noisy, however. To quickly focus on systematic reviews (knowing that you may be missing some), simply search for the word systematic in the title:
"neoadjuvant chemotherapy" AND systematic[ti]
Any PRISMA-compliant systematic review will be captured by this method since including the words "systematic review" in the title is a requirement of the PRISMA checklist. Cochrane systematic reviews do not include 'systematic' in the title, however. It's worth checking the Cochrane Database of Systematic Reviews independently.
You can also search for protocols that will indicate that another group has set out on a similar project. Many investigators will register their protocols in PROSPERO , a registry of review protocols. Other published protocols as well as Cochrane Review protocols appear in the Cochrane Methodology Register, a part of the Cochrane Library .
- Next: Guides and Standards >>
- Last Updated: Sep 25, 2024 2:45 PM
- URL: https://guides.library.harvard.edu/meta-analysis
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Study designs: Part 7 – Systematic reviews
Priya ranganathan, rakesh aggarwal.
- Author information
- Article notes
- Copyright and License information
Address for correspondence: Dr. Priya Ranganathan, Department of Anaesthesiology, Tata Memorial Centre, Homi Bhabha National Institute, Mumbai, Maharashtra, India. E-mail: [email protected]
Received 2020 Apr 4; Accepted 2020 Apr 8; Issue date 2020 Apr-Jun.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
In this series on research study designs, we have so far looked at different types of primary research designs which attempt to answer a specific question. In this segment, we discuss systematic review, which is a study design used to summarize the results of several primary research studies. Systematic reviews often also use meta-analysis, which is a statistical tool to mathematically collate the results of various research studies to obtain a pooled estimate of treatment effect; this will be discussed in the next article.
Keywords: Research design, review [publication type], systematic review [publication type]
In the previous six articles in this series on study designs, we have looked at different types of primary research study designs which are used to answer research questions. In this article, we describe the systematic review, a type of secondary research design that is used to summarize the results of prior primary research studies. Systematic reviews are considered the highest level of evidence for a particular research question.[ 1 ]
SYSTEMATIC REVIEWS
As defined in the Cochrane Handbook for Systematic Reviews of Interventions , “Systematic reviews seek to collate evidence that fits pre-specified eligibility criteria in order to answer a specific research question. They aim to minimize bias by using explicit, systematic methods documented in advance with a protocol.”[ 2 ]
NARRATIVE VERSUS SYSTEMATIC REVIEWS
Review of available data has been done since times immemorial. However, the traditional narrative reviews (“expert reviews”) do not involve a systematic search of the literature. Instead, the author of the review, usually an expert on the subject, used informal methods to identify (what he or she thinks are) the key studies on the topic. The final review thus is a summary of these “selected” studies. Since studies are chosen at will (haphazardly!) and without clearly defined criteria, such reviews preferentially include those studies that favor the author's views, leading to a potential for subjectivity or selection bias.
In contrast, systematic reviews involve a formal prespecified protocol with explicit, transparent criteria for the inclusion and exclusion of studies, thereby ensuring completeness of coverage of the available evidence, and providing a more objective, replicable, and comprehensive overview it.
META-ANALYSIS
Many systematic reviews use an additional tool, known as meta-analysis, which is a statistical technique for combining the results of multiple studies in a systematic review in a mathematically appropriate way, to create a single (pooled) and more precise estimate of treatment effect. The feasibility of performing a meta-analysis in a systematic review depends on the number of studies included in the final review and the degree of heterogeneity in the inclusion criteria as well as the results between the included studies. Meta-analysis will be discussed in detail in the next article in this series.
THE PROCESS OF A SYSTEMATIC REVIEW
The conduct of a systematic review involves several sequential key steps.[ 3 , 4 ] As in other research study designs, a clearly stated research question and a well-written research protocol are essential before commencing a systematic review.
Step 1: Stating the review question
Systematic reviews can be carried out in any field of medical research, e.g. efficacy or safety of interventions, diagnostics, screening or health economics. In this article, we focus on systematic reviews of studies looking at the efficacy of interventions. As for the other study designs, for a systematic review too, the question is best framed using the Population, Intervention, Comparator, and Outcome (PICO) format.
For example, Safi et al . carried out a systematic review on the effect of beta-blockers on the outcomes of patients with myocardial infarction.[ 5 ] In this review, the Population was patients with suspected or confirmed myocardial infarction, the Intervention was beta-blocker therapy, the Comparator was either placebo or no intervention, and the Outcomes were all-cause mortality and major adverse cardiovascular events. The review question was “ In patients with suspected or confirmed myocardial infarction, does the use of beta-blockers affect mortality or major adverse cardiovascular outcomes? ”
Step 2: Listing the eligibility criteria for studies to be included
It is essential to explicitly define a priori the criteria for selection of studies which will be included in the review. Besides the PICO components, some additional criteria used frequently for this purpose include language of publication (English versus non-English), publication status (published as full paper versus unpublished), study design (randomized versus quasi-experimental), age group (adults versus children), and publication year (e.g. in the last 5 years, or since a particular date). The PICO criteria used may not be very specific, e.g. it is possible to include studies that use one or the other drug belonging to the same group. For instance, the systematic review by Safi et al . included all randomized clinical trials, irrespective of setting, blinding, publication status, publication year, or language, and reported outcomes, that had used any beta-blocker and in a broad range of doses.[ 5 ]
Step 3: Comprehensive search for studies that meet the eligibility criteria
A thorough literature search is essential to identify all articles related to the research question and to ensure that no relevant article is left out. The search may include one or more electronic databases and trial registries; in addition, it is common to hand-search the cross-references in the articles identified through such searches. One could also plan to reach out to experts in the field to identify unpublished data, and to search the grey literature non-peer-reviewednon-peer-reviewed. This last option is particularly helpful non-pharmacologic (theses, conference abstracts, and non-peer-reviewed journals). These sources are particularly helpful when the intervention is relatively new, since data on these may not yet have been published as full papers and hence are unlikely to be found in literature databases. In the review by Safi et al ., the search strategy included not only several electronic databases (Cochrane, MEDLINE, EMBASE, LILACS, etc.) but also other resources (e.g. Google Scholar, WHO International Clinical Trial Registry Platform, and reference lists of identified studies).[ 5 ] It is not essential to include all the above databases in one's search. However, it is mandatory to define in advance which of these will be searched.
Step 4: Identifying and selecting relevant studies
Once the search strategy defined in the previous step has been run to identify potentially relevant studies, a two-step process is followed. First, the titles and abstracts of the identified studies are processed to exclude any duplicates and to discard obviously irrelevant studies. In the next step, full-text papers of the remaining articles are retrieved and closely reviewed to identify studies that meet the eligibility criteria. To minimize bias, these selection steps are usually performed independently by at least two reviewers, who also assign a reason for non-selection to each discarded study. Any discrepancies are then resolved either by an independent reviewer or by mutual consensus of the original reviewers. In the Cochrane review on beta-blockers referred to above, two review authors independently screened the titles for inclusion, and then, four review authors independently reviewed the screen-positive studies to identify the trials to be included in the final review.[ 5 ] Disagreements were resolved by discussion or by taking the opinion of a separate reviewer. A summary of this selection process, showing the degree of agreement between reviewers, and a flow diagram that depicts the numbers of screened, included and excluded (with reason for exclusion) studies are often included in the final review.
Step 5: Data extraction
In this step, from each selected study, relevant data are extracted. This should be done by at least two reviewers independently, and the data then compared to identify any errors in extraction. Standard data extraction forms help in objective data extraction. The data extracted usually contain the name of the author, the year of publication, details of intervention and control treatments, and the number of participants and outcome data in each group. In the review by Safi et al ., four review authors independently extracted data and resolved any differences by discussion.[ 5 ]
Handling missing data
Some of the studies included in the review may not report outcomes in accordance with the review methodology. Such missing data can be handled in two ways – by contacting authors of the original study to obtain the necessary data and by using data imputation techniques. Safi et al . used both these approaches – they tried to get data from the trial authors; however, where that failed, they analyzed the primary outcome (mortality) using the best case (i.e. presuming that all the participants in the experimental arm with missing data had survived and those in the control arm with missing mortality data had died – representing the maximum beneficial effect of the intervention) and the worst case (all the participants with missing data in the experimental arm assumed to have died and those in the control arm to have survived – representing the least beneficial effect of the intervention) scenarios.
Evaluating the quality (or risk of bias) in the included studies
The overall quality of a systematic review depends on the quality of each of the included studies. Quality of a study is inversely proportional to the potential for bias in its design. In our previous articles on interventional study design in this series, we discussed various methods to reduce bias – such as randomization, allocation concealment, participant and assessor blinding, using objective endpoints, minimizing missing data, the use of intention-to-treat analysis, and complete reporting of all outcomes.[ 6 , 7 ] These features form the basis of the Cochrane Risk of Bias Tool (RoB 2), which is a commonly used instrument to assess the risk of bias in the studies included in a systematic review.[ 8 ] Based on this tool, one can classify each study in a review as having low risk of bias, having some concerns regarding bias, or at high risk of bias. Safi et al . used this tool to classify the included studies as having low or high risk of bias and presented these data in both tabular and graphical formats.[ 5 ]
In some reviews, the authors decide to summarize only studies with a low risk of bias and to exclude those with a high risk of bias. Alternatively, some authors undertake a separate analysis of studies with low risk of bias, besides an analysis of all the studies taken together. The conclusions from such analyses of only high-quality studies may be more robust.
Step 6: Synthesis of results
The data extracted from various studies are pooled quantitatively (known as a meta-analysis) or qualitatively (if pooling of results is not considered feasible). For qualitative reviews, data are usually presented in the tabular format, showing the characteristics of each included study, to allow for easier interpretation.
Sensitivity analyses
Sensitivity analyses are used to test the robustness of the results of a systematic review by examining the impact of excluding or including studies with certain characteristics. As referred to above, this can be based on the risk of bias (methodological quality), studies with a specific study design, studies with a certain dosage or schedule, or sample size. If results of these different analyses are more-or-less the same, one can be more certain of the validity of the findings of the review. Furthermore, such analyses can help identify whether the effect of the intervention could vary across different levels of another factor. In the beta-blocker review, sensitivity analysis was performed depending on the risk of bias of included studies.[ 5 ]
IMPORTANT RESOURCES FOR CARRYING OUT SYSTEMATIC REVIEWS AND META-ANALYSES
Cochrane is an organization that works to produce good-quality, updated systematic reviews related to human healthcare and policy, which are accessible to people across the world.[ 9 ] There are more than 7000 Cochrane reviews on various topics. One of its main resources is the Cochrane Library (available at https://www.cochranelibrary.com/ ), which incorporates several databases with different types of high-quality evidence to inform healthcare decisions, including the Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), and Cochrane Clinical Answers.
The Cochrane Handbook for Systematic Reviews of Interventions
The Cochrane handbook is an official guide, prepared by the Cochrane Collaboration, to the process of preparing and maintaining Cochrane systematic reviews.[ 10 ]
Review Manager software
Review Manager (RevMan) is a software developed by Cochrane to support the preparation and maintenance of systematic reviews, including tools for performing meta-analysis.[ 11 ] It is freely available in both online (RevMan Web) and offline (RevMan 5.3) versions.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement is an evidence-based minimum set of items for reporting of systematic reviews and meta-analyses of randomized trials.[ 12 ] It can be used both by authors of such studies to improve the completeness of reporting and by reviewers and readers to critically appraise a systematic review. There are several extensions to the PRISMA statement for specific types of reviews. An update is currently underway.
Meta-analysis of Observational Studies in Epidemiology statement
The Meta-analysis of Observational Studies in Epidemiology statement summarizes the recommendations for reporting of meta-analyses in epidemiology.[ 13 ]
PROSPERO is an international database for prospective registration of protocols for systematic reviews in healthcare.[ 14 ] It aims to avoid duplication of and to improve transparency in reporting of results of such reviews.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- 1. Centre for Evidence Based Medicine. [Last accessed on 2020 Apr 01]. Available from: http://wwwcebmnet .
- 2. Chandler J, Cumpston M, Thomas J, Higgins JP, Deeks JJ, Clarke MJ. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Introduction. Cochrane Handbook for Systematic Reviews of Interventions Version 60 (updated August 2019) Ch 1 Cochrane. 2019. Available from: http://wwwtrainingcochraneorg/handbook .
- 3. Impellizzeri FM, Bizzini M. Systematic review and meta-analysis: A primer. Int J Sports Phys Ther. 2012;7:493–503. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Akobeng AK. Understanding systematic reviews and meta-analysis. Arch Dis Child. 2005;90:845–8. doi: 10.1136/adc.2004.058230. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Safi S, Sethi NJ, Nielsen EE, Feinberg J, Jakobsen JC, Gluud C. Beta-blockers for suspected or diagnosed acute myocardial infarction. Cochrane Database Syst Rev. 2019;12:CD012484. doi: 10.1002/14651858.CD012484.pub2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Aggarwal R, Ranganathan P. Study designs: Part 5 – Interventional studies (II) Perspect Clin Res. 2019;10:183–6. doi: 10.4103/picr.PICR_138_19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Ranganathan P, Aggarwal R. Study designs: Part 6 – Interventional studies (III) Perspect Clin Res. 2020;11:47–50. doi: 10.4103/picr.PICR_209_19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. RoB 2: A Revised Cochrane Risk-Of-Bias Tool for Randomized Trials. [Last accessed on 2020 Apr 01]. Available from: https://methodscochraneorg/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials .
- 9. Cochrane. [Last accessed on 2020 Apr 03]. Available from: https://workcochraneorg/organisation-known-cochrane .
- 10. The Cochrane Handbook for Systematic Reviews of Interventions. [Last accessed on 2020 Apr 01]. Available from: https://cochrane-handbookorg .
- 11. Cochrane RevMan. [Last accessed on 2020 Apr 01]. Available from: https://trainingcochraneorg/online-learning/core-software-cochrane-reviews/revman .
- 12. PRISMA Statement. [Last accessed on 2020 Apr 01]. Available from: http://wwwprisma-statementorg/
- 13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. PROSPERO: International Prospective Register of Systematic Reviews. [Last accessed on 2020 Apr 01]. Available from: https://wwwcrdyorkacuk/PROSPERO/
- View on publisher site
- PDF (485.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Easy guide to conducting a systematic review
Affiliations.
- 1 Discipline of Child and Adolescent Health, University of Sydney, Sydney, New South Wales, Australia.
- 2 Department of Nephrology, The Children's Hospital at Westmead, Sydney, New South Wales, Australia.
- 3 Education Department, The Children's Hospital at Westmead, Sydney, New South Wales, Australia.
- PMID: 32364273
- DOI: 10.1111/jpc.14853
A systematic review is a type of study that synthesises research that has been conducted on a particular topic. Systematic reviews are considered to provide the highest level of evidence on the hierarchy of evidence pyramid. Systematic reviews are conducted following rigorous research methodology. To minimise bias, systematic reviews utilise a predefined search strategy to identify and appraise all available published literature on a specific topic. The meticulous nature of the systematic review research methodology differentiates a systematic review from a narrative review (literature review or authoritative review). This paper provides a brief step by step summary of how to conduct a systematic review, which may be of interest for clinicians and researchers.
Keywords: research; research design; systematic review.
© 2020 Paediatrics and Child Health Division (The Royal Australasian College of Physicians).
Publication types
- Systematic Review
- Research Design*
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- Systematic Review | Definition, Examples & Guide
Systematic Review | Definition, Examples & Guide
Published on 15 June 2022 by Shaun Turney . Revised on 18 July 2024.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesise all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer.
They answered the question ‘What is the effectiveness of probiotics in reducing eczema symptoms and improving quality of life in patients with eczema?’
In this context, a probiotic is a health product that contains live microorganisms and is taken by mouth. Eczema is a common skin condition that causes red, itchy skin.
Table of contents
What is a systematic review, systematic review vs meta-analysis, systematic review vs literature review, systematic review vs scoping review, when to conduct a systematic review, pros and cons of systematic reviews, step-by-step example of a systematic review, frequently asked questions about systematic reviews.
A review is an overview of the research that’s already been completed on a topic.
What makes a systematic review different from other types of reviews is that the research methods are designed to reduce research bias . The methods are repeatable , and the approach is formal and systematic:
- Formulate a research question
- Develop a protocol
- Search for all relevant studies
- Apply the selection criteria
- Extract the data
- Synthesise the data
- Write and publish a report
Although multiple sets of guidelines exist, the Cochrane Handbook for Systematic Reviews is among the most widely used. It provides detailed guidelines on how to complete each step of the systematic review process.
Systematic reviews are most commonly used in medical and public health research, but they can also be found in other disciplines.
Systematic reviews typically answer their research question by synthesising all available evidence and evaluating the quality of the evidence. Synthesising means bringing together different information to tell a single, cohesive story. The synthesis can be narrative ( qualitative ), quantitative , or both.
Prevent plagiarism, run a free check.
Systematic reviews often quantitatively synthesise the evidence using a meta-analysis . A meta-analysis is a statistical analysis, not a type of review.
A meta-analysis is a technique to synthesise results from multiple studies. It’s a statistical analysis that combines the results of two or more studies, usually to estimate an effect size .
A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarise and evaluate previous work, without using a formal, explicit method.
Although literature reviews are often less time-consuming and can be insightful or helpful, they have a higher risk of bias and are less transparent than systematic reviews.
Similar to a systematic review, a scoping review is a type of review that tries to minimise bias by using transparent and repeatable methods.
However, a scoping review isn’t a type of systematic review. The most important difference is the goal: rather than answering a specific question, a scoping review explores a topic. The researcher tries to identify the main concepts, theories, and evidence, as well as gaps in the current research.
Sometimes scoping reviews are an exploratory preparation step for a systematic review, and sometimes they are a standalone project.
A systematic review is a good choice of review if you want to answer a question about the effectiveness of an intervention , such as a medical treatment.
To conduct a systematic review, you’ll need the following:
- A precise question , usually about the effectiveness of an intervention. The question needs to be about a topic that’s previously been studied by multiple researchers. If there’s no previous research, there’s nothing to review.
- If you’re doing a systematic review on your own (e.g., for a research paper or thesis), you should take appropriate measures to ensure the validity and reliability of your research.
- Access to databases and journal archives. Often, your educational institution provides you with access.
- Time. A professional systematic review is a time-consuming process: it will take the lead author about six months of full-time work. If you’re a student, you should narrow the scope of your systematic review and stick to a tight schedule.
- Bibliographic, word-processing, spreadsheet, and statistical software . For example, you could use EndNote, Microsoft Word, Excel, and SPSS.
A systematic review has many pros .
- They minimise research b ias by considering all available evidence and evaluating each study for bias.
- Their methods are transparent , so they can be scrutinised by others.
- They’re thorough : they summarise all available evidence.
- They can be replicated and updated by others.
Systematic reviews also have a few cons .
- They’re time-consuming .
- They’re narrow in scope : they only answer the precise research question.
The 7 steps for conducting a systematic review are explained with an example.
Step 1: Formulate a research question
Formulating the research question is probably the most important step of a systematic review. A clear research question will:
- Allow you to more effectively communicate your research to other researchers and practitioners
- Guide your decisions as you plan and conduct your systematic review
A good research question for a systematic review has four components, which you can remember with the acronym PICO :
- Population(s) or problem(s)
- Intervention(s)
- Comparison(s)
You can rearrange these four components to write your research question:
- What is the effectiveness of I versus C for O in P ?
Sometimes, you may want to include a fourth component, the type of study design . In this case, the acronym is PICOT .
- Type of study design(s)
- The population of patients with eczema
- The intervention of probiotics
- In comparison to no treatment, placebo , or non-probiotic treatment
- The outcome of changes in participant-, parent-, and doctor-rated symptoms of eczema and quality of life
- Randomised control trials, a type of study design
Their research question was:
- What is the effectiveness of probiotics versus no treatment, a placebo, or a non-probiotic treatment for reducing eczema symptoms and improving quality of life in patients with eczema?
Step 2: Develop a protocol
A protocol is a document that contains your research plan for the systematic review. This is an important step because having a plan allows you to work more efficiently and reduces bias.
Your protocol should include the following components:
- Background information : Provide the context of the research question, including why it’s important.
- Research objective(s) : Rephrase your research question as an objective.
- Selection criteria: State how you’ll decide which studies to include or exclude from your review.
- Search strategy: Discuss your plan for finding studies.
- Analysis: Explain what information you’ll collect from the studies and how you’ll synthesise the data.
If you’re a professional seeking to publish your review, it’s a good idea to bring together an advisory committee . This is a group of about six people who have experience in the topic you’re researching. They can help you make decisions about your protocol.
It’s highly recommended to register your protocol. Registering your protocol means submitting it to a database such as PROSPERO or ClinicalTrials.gov .
Step 3: Search for all relevant studies
Searching for relevant studies is the most time-consuming step of a systematic review.
To reduce bias, it’s important to search for relevant studies very thoroughly. Your strategy will depend on your field and your research question, but sources generally fall into these four categories:
- Databases: Search multiple databases of peer-reviewed literature, such as PubMed or Scopus . Think carefully about how to phrase your search terms and include multiple synonyms of each word. Use Boolean operators if relevant.
- Handsearching: In addition to searching the primary sources using databases, you’ll also need to search manually. One strategy is to scan relevant journals or conference proceedings. Another strategy is to scan the reference lists of relevant studies.
- Grey literature: Grey literature includes documents produced by governments, universities, and other institutions that aren’t published by traditional publishers. Graduate student theses are an important type of grey literature, which you can search using the Networked Digital Library of Theses and Dissertations (NDLTD) . In medicine, clinical trial registries are another important type of grey literature.
- Experts: Contact experts in the field to ask if they have unpublished studies that should be included in your review.
At this stage of your review, you won’t read the articles yet. Simply save any potentially relevant citations using bibliographic software, such as Scribbr’s APA or MLA Generator .
- Databases: EMBASE, PsycINFO, AMED, LILACS, and ISI Web of Science
- Handsearch: Conference proceedings and reference lists of articles
- Grey literature: The Cochrane Library, the metaRegister of Controlled Trials, and the Ongoing Skin Trials Register
- Experts: Authors of unpublished registered trials, pharmaceutical companies, and manufacturers of probiotics
Step 4: Apply the selection criteria
Applying the selection criteria is a three-person job. Two of you will independently read the studies and decide which to include in your review based on the selection criteria you established in your protocol . The third person’s job is to break any ties.
To increase inter-rater reliability , ensure that everyone thoroughly understands the selection criteria before you begin.
If you’re writing a systematic review as a student for an assignment, you might not have a team. In this case, you’ll have to apply the selection criteria on your own; you can mention this as a limitation in your paper’s discussion.
You should apply the selection criteria in two phases:
- Based on the titles and abstracts : Decide whether each article potentially meets the selection criteria based on the information provided in the abstracts.
- Based on the full texts: Download the articles that weren’t excluded during the first phase. If an article isn’t available online or through your library, you may need to contact the authors to ask for a copy. Read the articles and decide which articles meet the selection criteria.
It’s very important to keep a meticulous record of why you included or excluded each article. When the selection process is complete, you can summarise what you did using a PRISMA flow diagram .
Next, Boyle and colleagues found the full texts for each of the remaining studies. Boyle and Tang read through the articles to decide if any more studies needed to be excluded based on the selection criteria.
When Boyle and Tang disagreed about whether a study should be excluded, they discussed it with Varigos until the three researchers came to an agreement.
Step 5: Extract the data
Extracting the data means collecting information from the selected studies in a systematic way. There are two types of information you need to collect from each study:
- Information about the study’s methods and results . The exact information will depend on your research question, but it might include the year, study design , sample size, context, research findings , and conclusions. If any data are missing, you’ll need to contact the study’s authors.
- Your judgement of the quality of the evidence, including risk of bias .
You should collect this information using forms. You can find sample forms in The Registry of Methods and Tools for Evidence-Informed Decision Making and the Grading of Recommendations, Assessment, Development and Evaluations Working Group .
Extracting the data is also a three-person job. Two people should do this step independently, and the third person will resolve any disagreements.
They also collected data about possible sources of bias, such as how the study participants were randomised into the control and treatment groups.
Step 6: Synthesise the data
Synthesising the data means bringing together the information you collected into a single, cohesive story. There are two main approaches to synthesising the data:
- Narrative ( qualitative ): Summarise the information in words. You’ll need to discuss the studies and assess their overall quality.
- Quantitative : Use statistical methods to summarise and compare data from different studies. The most common quantitative approach is a meta-analysis , which allows you to combine results from multiple studies into a summary result.
Generally, you should use both approaches together whenever possible. If you don’t have enough data, or the data from different studies aren’t comparable, then you can take just a narrative approach. However, you should justify why a quantitative approach wasn’t possible.
Boyle and colleagues also divided the studies into subgroups, such as studies about babies, children, and adults, and analysed the effect sizes within each group.
Step 7: Write and publish a report
The purpose of writing a systematic review article is to share the answer to your research question and explain how you arrived at this answer.
Your article should include the following sections:
- Abstract : A summary of the review
- Introduction : Including the rationale and objectives
- Methods : Including the selection criteria, search method, data extraction method, and synthesis method
- Results : Including results of the search and selection process, study characteristics, risk of bias in the studies, and synthesis results
- Discussion : Including interpretation of the results and limitations of the review
- Conclusion : The answer to your research question and implications for practice, policy, or research
To verify that your report includes everything it needs, you can use the PRISMA checklist .
Once your report is written, you can publish it in a systematic review database, such as the Cochrane Database of Systematic Reviews , and/or in a peer-reviewed journal.
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a dissertation , thesis, research paper , or proposal .
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarise yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Turney, S. (2024, July 17). Systematic Review | Definition, Examples & Guide. Scribbr. Retrieved 21 October 2024, from https://www.scribbr.co.uk/research-methods/systematic-reviews/
Is this article helpful?


Shaun Turney
Other students also liked, what is a literature review | guide, template, & examples, exploratory research | definition, guide, & examples, what is peer review | types & examples.
Doing a Systematic Review: A Student’s Guide
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
What is Systematic Review?
A systematic review is a comprehensive, structured analysis of existing research on a specific topic. It uses predefined criteria to identify, evaluate, and synthesize relevant studies, aiming to provide an unbiased summary of the current evidence.
The explicit and systematic approach of a systematic review distinguishes it from traditional reviews and commentaries.
How systematic reviews differ from narrative reviews:
- Goals: Narrative reviews provide a summary or overview of a topic, while systematic reviews answer a focused review question.
- Sources of Literature: Narrative reviews often use a non-exhaustive and unstated body of literature, which can lead to publication bias. Systematic reviews consider a list of databases, grey literature, and other sources.
- Selection Criteria: Narrative reviews usually use subjective or no selection criteria, which can lead to selection bias. Systematic reviews have a clear and explicit selection process.
- Appraisal of Study Quality: Narrative reviews vary in their evaluation of study quality. Systematic reviews use standard checklists for a rigorous appraisal of study quality.
Systematic reviews are time-intensive and need a research team with multiple skills and contributions. There are some cases where systematic reviews are unable to meet the necessary objectives of the review question.
In these cases, scoping reviews (which are sometimes called scoping exercises/scoping studies) may be more useful to consider.
Scoping reviews are different from systematic reviews because they may not include a mandatory critical appraisal of the included studies or synthesize the findings from individual studies.

Assessing The Need For A Systematic Review
When assessing the need for a systematic review, one must first check if any existing or ongoing reviews already exist and determine if a new review is justified.
Scoping reviews frequently serve as preliminary steps before conducting full systematic reviews. They help assess the available literature’s breadth, identify key concepts, and determine the feasibility of a more comprehensive review.
This initial exploration guides researchers in refining their approach for subsequent in-depth analyses.
This process should begin by searching relevant databases.
Resources to consider searching include:
- NICE : National Institute for Health and Clinical Excellence
- Campbell Library of Systematic Reviews for reviews in education, crime and justice, and social welfare
- EPPI : Evidence for Policy and Practice Information Centre, particularly their database of systematic and non-systematic reviews of public health interventions (DoPHER)
- MEDLINE : Primarily covers the medical domain, making it a primary resource for systematic reviews concerning healthcare interventions
- PsycINFO : For research in psychology, psychiatry, behavioral sciences, and social sciences
- Cochrane Library (specifically CDSR) : Focuses on systematic reviews of health care interventions, providing regularly updated and critically appraised reviews
If an existing review addressing the question of interest is found, its quality should be assessed to determine its suitability for guiding policy and practice.
If a high-quality, relevant review is located, but its completion date is some time ago, updating the review might be warranted.
Assessing current relevance is vital, especially in rapidly evolving research fields. Collaboration with the original research team might be beneficial during the update process, as they could provide access to their data.
If the review is deemed to be of adequate quality and remains relevant, undertaking another systematic review may not be necessary.
When a new systematic review or an update is deemed necessary, the subsequent step involves establishing a review team and potentially an advisory group, who will then develop the review protocol.
How To Conduct A Systematic Review
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is a reporting guideline designed to improve the transparency and completeness of systematic review reporting.
PRISMA was created to tackle the issue of inadequate reporting often found in systematic reviews:
- Checklist : PRISMA features a 27-item checklist covering all aspects of a systematic review, from the rationale and objectives to the synthesis of findings and discussion of limitations. Each checklist item is accompanied by detailed reporting recommendations in an Explanation and Elaboration document .
- Flow Diagram : PRISMA also includes a flow diagram to visually represent the study selection process, offering a clear, standardized way to illustrate how researchers arrived at the final set of included studies.

Step 1: write a research protocol
A protocol in the context of systematic reviews is a detailed plan that outlines the methodology to be employed throughout the review process.
The protocol serves as a roadmap, guiding researchers through each stage of the review in a transparent and replicable manner.
This document should provide specific details about every stage of the research process, including the methodology for identifying, selecting, and analyzing relevant studies.
For example, the protocol should specify search strategies for relevant studies, including whether the search will encompass unpublished works.
The protocol should be created before beginning the research process to ensure transparency and reproducibility.
This pre-determined plan ensures that decisions made during the review are objective and free from bias, as they are based on pre-established criteria.
Protocol modifications are sometimes necessary during systematic reviews. While adhering to the protocol is crucial for minimizing bias, there are instances where modifications are justified. For instance, a deeper understanding of the research question that emerges from examining primary research might necessitate changes to the protocol.
Systematic reviews should be registered at inception (at the protocol stage) for these reasons:
- To help avoid unplanned duplication
- To enable the comparison of reported review methods with what was planned in the protocol
This registration prevents duplication (research waste) and makes the process easy when the full systematic review is sent for publication.
PROSPERO is an international database of prospectively registered systematic reviews in health and social care. Non-Cochrane protocols should be registered on PROSPERO.
Research Protocol
Rasika Jayasekara, Nicholas Procter. The effects of cognitive behaviour therapy for major depression in older adults: a systematic review. PROSPERO 2012 CRD42012003151 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42012003151
Review question
How effective is CBT compared with other interventions, placebo or standard treatment in achieving relapse prevention and improving mental status for older adults with major depression?
The search strategy aims to find both published and unpublished studies and publications. The search will be limited to English language papers published from 2002 to 2012.
A three-step search strategy will be developed using MeSH terminology and keywords to ensure that all materials relevant to the review are captured.
An initial limited search of MEDLINE and CINAHL will be undertaken followed by an analysis of the text words contained in the title and abstract, and of the index terms used to describe the article. A second search using all identified keywords and index terms will then be undertaken.
Thirdly, the reference list of all identified reports and articles will be searched for additional studies.
The databases to be searched included:
- Cochrane Central Register of Controlled Trials
- Controlled Trials
- Current Contents
The search for unpublished studies will include:
- Digital Dissertations (Proquest)
- Conference Proceedings
Experts in the field will be contacted for ongoing and unpublished trials. Experts will be identified through journal publications.
Types of study to be included
All randomised controlled trials (RCTs) assessing the effectiveness of CBT as a treatment for older adults with major depression when compared to standard care, specific medication, other therapies and no intervention will be considered.
In the absence of RCTs, other research designs such as quasi-experimental studies, case-controlled studies and cohort studies will be examined. However, descriptive studies and expert opinion will be excluded.
Condition or domain being studied
Major depression is diagnosed according to DSM IV or ICD 10 criteria.
Where trials fail to employ diagnostic criteria, the severity of depression will be described by the use of standardised rating scales, including the Hamilton Depression Rating Scale, Montgomery and Asberg Rating Scale and the Geriatric Depression Rating Scale.
The trials including participants with an explicit diagnosis of dementia or Parkinson’s disease and other mental illnesses will be excluded.
The review will include trials conducted in primary, secondary, community, nursing homes and in-patient settings.
Participants/population
The review will include trials in which patients are described as elderly, geriatric, or older adults, or in which all patients will be aged 55 or over (many North American trials of older adult populations use a cut-off of 55 years).
The review will include trials with subjects of either sex. Where possible, participants will be categorised as community or long term care residents.
Intervention(s), exposure(s)
The review will focus on interventions designed to assess the effects of CBT for older adults with major depression.
The label cognitive behavioural therapy has been applied to a variety of interventions and, accordingly, it is difficult to provide a single, unambiguous definition.
In order to be classified as CBT the intervention must clearly demonstrate the following components:
- the intervention involves the recipient establishing links between their thoughts, feelings and actions with respect to the target symptom;
- the intervention involves the correction of the person’s misperceptions, irrational beliefs and reasoning biases related to the target symptom.
- – the recipient monitoring his or her own thoughts, feelings and behaviours with respect to the target symptom; and
- – the promotion of alternative ways of coping with the target symptom.
In addition, all therapies that do not meet these criteria (or that provide insufficient information) but are labelled as ‘CBT’ or ‘Cognitive Therapy’ will be included as ‘less well defined’ CBT.
Comparator(s)/control
other interventions, placebo or standard treatment
Main outcome(s)
Primary outcomes
- Depression level as assessed by Hamilton Depression Rating Scale, Montgomery or Asberg Rating Scale or the Geriatric Depression Rating Scale.
- Relapse (as defined in the individual studies)
- Death (sudden, unexpected death or suicide).
- Psychological well being (as defined in the individual studies)
Measures of effect
The review will categorise outcomes into those measured in the shorter term (within 12 weeks of the onset of therapy), medium term (within 13 to 26 weeks of the onset of therapy) and longer term (over 26 weeks since the onset of therapy).
Additional outcome(s)
Secondary outcomes
- Mental state
- Quality of life
- Social functioning
- Hospital readmission
- Unexpected or unwanted effect (adverse effects), such as anxiety, depression and dependence on the relationship with the therapist
Data extraction (selection and coding)
Data will be extracted from papers included in the review using JBI-MAStARI. In this stage, any relevant studies will be extracted in relation to their population, interventions, study methods and outcomes.
Where data are missing or unclear, authors will be contacted to obtain information.
Risk of bias (quality) assessment
All papers selected for retrieval will be assessed by two independent reviewers for methodological validity prior to inclusion in the review.
Since the review will evaluate the experimental studies only, The Joanna Briggs Institute Meta Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI) will be used to evaluate each study’s methodological validity.
If there is a disagreement between the two reviewers, there will be a discussion with the third reviewer to solve the dissimilarity.
Strategy for data synthesis
Where possible quantitative research study results will be pooled in statistical meta-analysis using Review Manager Software from the Cochrane Collaboration.
Odds ratio (for categorical outcome data) or standardised mean differences (for continuous data) and their 95% confidence intervals will be calculated for each study.
Heterogeneity will be assessed using the standard Chi-square. Where statistical pooling is not possible the findings will be presented in narrative form.
Step 2: formulate a research question
Developing a focused research question is crucial for a systematic review, as it underpins every stage of the review process.
The question defines the review’s nature and scope, guides the identification of relevant studies, and shapes the data extraction and synthesis processes.
It’s essential that the research question is answerable and clearly stated in the review protocol, ensuring that the review’s boundaries are well-defined.
A narrow question may limit the number of relevant studies and generalizability, while a broad question can make it challenging to reach specific conclusions.
PICO Framework
The PICO framework is a model for creating focused clinical research questions. The acronym PICO stands for:
- P opulation/Patient/Problem: This element defines the specific group of people the research question pertains to.
- I ntervention: This is the treatment, test, or exposure being considered for the population.
- C omparison: This is the alternative intervention or control group against which the intervention is being compared.
- O utcome: This element specifies the results or effects of the interventions being investigated
Using the PICO format when designing research helps to minimize bias because the questions and methods of the review are formulated before reviewing any literature.
The PICO elements are also helpful in defining the inclusion criteria used to select sources for the systematic review.
The PICO framework is commonly employed in systematic reviews that primarily analyze data from randomized controlled trials .
Not every element of PICO is required for every research question. For instance, it is not always necessary to have a comparison
Types of questions that can be answered using PICO:
“In patients with a recent acute stroke (less than 6 weeks) with reduced mobility ( P ), is any specific physiotherapy approach ( I ) more beneficial than no physiotherapy ( C ) at improving independence in activities of daily living and gait speed ( O )?
“For women who have experienced domestic violence ( P ), how effective are advocacy programmes ( I ) compared to other treatments ( C ) on improving the quality of life ( O )?”
Etiology/Harm
Are women with a history of pelvic inflammatory disease (PID) ( P ) at higher risk for gynecological cancers ( O ) than women with no history of PID ( C )?
Among asymptomatic adults at low risk of colon cancer ( P ), is fecal immunochemical testing (FIT) ( I ) as sensitive and specific for diagnosing colon cancer ( O ) as colonoscopy ( C )?
Among adults with pneumonia ( P ), do those with chronic kidney disease (CKD) ( I ) have a higher mortality rate ( O ) than those without CKD ( C )?
Alternative Frameworks
- PICOCS : This framework, used in public health research, adds a “ C ontext” element to the PICO framework. This is useful for examining how the environment or setting in which an intervention is delivered might influence its effectiveness.
- PICOC : This framework expands on PICO by incorporating “ C osts” as an element of the research question. It is particularly relevant to research questions involving economic evaluations of interventions.
- ECLIPSE : E xpectations, C lient group, L ocation, I mpact, P rofessionals involved, S ervice, and E valuation. It is a mnemonic device designed to aid in searching for health policy and management information.
- PEO : This acronym, standing for P atient, E xposure, and O utcome, is a variation of PICO used when the research question focuses on the relationship between exposure to a risk factor and a specific outcome.
- PIRD : This acronym stands for P opulation, I ndex Test, R eference Test, and D iagnosis of Interest, guiding research questions that focus on evaluating the diagnostic accuracy of a particular test.
- PFO : This acronym, representing P opulation, P rognostic F actors, and O utcome, is tailored for research questions that aim to investigate the relationship between specific prognostic factors and a particular health outcome.
- SDMO : This framework, which stands for S tudies, D ata, M ethods, and O utcomes, assists in structuring research questions focused on methodological aspects of research, examining the impact of different research methods or designs on the quality of research findings.
Step 3: Search Strategy
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) provide appropriate guidance for reporting quantitative literature searches.
Present the full search strategies for all databases, registers and websites, including any filters and limits used. PRISMA 2020 Checklist
A search strategy is a comprehensive and reproducible plan for identifying all relevant research studies that address a specific research question.
This systematic approach to searching helps minimize bias and distinguishes systematic reviews from other types of literature reviews.
It’s important to be transparent about the search strategy and document all decisions for auditability. The goal is to identify all potentially relevant studies for consideration.
Here’s a breakdown of a search strategy:
Search String Construction
It is recommended to consult topic experts on the review team and advisory board in order to create as complete a list of search terms as possible for each concept.
To retrieve the most relevant results, a search string is used. This string is made up of:
- Keywords: Search terms should be relevant to the subject areas of the research question and should be identified for all components of the research question (e.g., Population, Intervention, Comparator, and Outcomes – PICO). Using relevant keywords helps minimize irrelevant search returns. Sources such as dictionaries, textbooks, and published articles can help identify appropriate keywords.
- Synonyms: These are words or phrases with similar meanings to the keywords, as authors may use different terms to describe the same concepts. Including synonyms helps cover variations in terminology and increases the chances of finding all relevant studies. For example, a drug intervention may be referred to by its generic name or by one of its several proprietary names.
- Truncation symbols : These broaden the search by capturing variations of a keyword. They function by locating every word that begins with a specific root. For example, if a user was researching interventions for smoking, they might use a truncation symbol to search for “smok*” to retrieve records with the words “smoke,” “smoker,” “smoking,” or “smokes.” This can save time and effort by eliminating the need to input every variation of a word into a database.
- Boolean operators: The use of Boolean operators (AND/OR/NEAR/NOT) helps to combine these terms effectively, ensuring that the search strategy is both sensitive and specific. For instance, using “AND” narrows the search to include only results containing both terms, while “OR” expands it to include results containing either term.
Information Sources
The primary goal is to find all published and unpublished studies that meet the predefined criteria of the research question. This includes considering various sources beyond typical databases
Information sources for systematic reviews can include a wide range of resources like scholarly databases, unpublished literature, conference papers, books, and even expert consultations.
Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. PRISMA 2020 Checklist
An exhaustive, systematic search strategy is developed with the assistance of an expert librarian.
- Electronic Databases : Searches should include seven key databases: CINAHL, Medline, APA PsycArticles, Psychology and Behavioral Sciences Collection, APA PsycInfo, SocINDEX with Full Text, and Web of Science: Core Collections.
- Grey Literature : In addition to databases, forensic or ‘expansive’ searches can be conducted. This includes: grey literature database searches (e.g. OpenGrey , WorldCat , Ethos ), conference proceedings, unpublished reports, theses , clinical trial databases , searches by names of authors of relevant publications. Independent research bodies may also be good sources of material, e.g. Centre for Research in Ethnic Relations , Joseph Rowntree Foundation , Carers UK .
- Citation Searching : Reference lists often lead to highly cited and influential papers in the field, providing valuable context and background information for the review.
- Handsearching : Manually searching through specific journals or conference proceedings page-by-page is another way to ensure all relevant studies are captured, particularly those not yet indexed in databases.
- Contacting Experts : Reaching out to researchers or experts in the field can provide access to unpublished data or ongoing research not yet publicly available.
It is important to note that this may not be an exhaustive list of all potential databases.
A systematic computerized search was performed for publications that appeared between 1974 and 2018 in English language journals. Four databases were searched including PsychINFO, Embase, OVOID MEDLINE, and AMED. The databases were searched with combinations of search terms relating to attachment (“attachment” OR “working model” OR “safe haven” OR “secure base” OR “felt security”) AND romantic couples (“dyad” OR “couple” OR “spous” OR “partner” OR “romantic” OR “wife” OR “husband” OR “close relationship” OR “interpersonal” OR “intimate” OR “mari”) AND social support (“support prov” OR “caregiving” OR “support giv” OR “social support” OR “enacted support” OR “support received” OR “receiv* support” OR “prov support” OR “dyadic coping” OR “interpersonal coping” OR “collaborative coping” OR “help‐seeking” OR “emotional support” OR “tangible support” OR “instrumental support” OR “perceived support” OR “responsive” OR “buffer” OR “partner support” OR “Support avail*” OR “available support”). The reference lists of the retrieved studies were checked to find other relevant publications, which were not identified in the computerized database searches.
Inclusion Criteria
Specify the inclusion and exclusion criteria for the review. PRISMA 2020 Checklist
Before beginning the literature search, researchers should establish clear eligibility criteria for study inclusion.
Inclusion criteria are used to select studies for a systematic review and should be based on the study’s research method and PICO elements.
To maintain transparency and minimize bias, eligibility criteria for study inclusion should be established a priori. Ideally, researchers should aim to include only high-quality randomized controlled trials that adhere to the intention-to-treat principle.
The selection of studies should not be arbitrary, and the rationale behind inclusion and exclusion criteria should be clearly articulated in the research protocol.
When specifying the inclusion and exclusion criteria, consider the following aspects:
- Intervention Characteristics: Researchers might decide that, in order to be included in the review, an intervention must have specific characteristics. They might require the intervention to last for a certain length of time, or they might determine that only interventions with a specific theoretical basis are appropriate for their review.
- Population Characteristics: A systematic review might focus on the effects of an intervention for a specific population. For instance, researchers might choose to focus on studies that included only nurses or physicians.
- Outcome Measures: Researchers might choose to include only studies that used outcome measures that met a specific standard.
- Age of Participants: If a systematic review is examining the effects of a treatment or intervention for children, the authors of the review will likely choose to exclude any studies that did not include children in the target age range.
- Diagnostic Status of Participants: Researchers conducting a systematic review of treatments for anxiety will likely exclude any studies where the participants were not diagnosed with an anxiety disorder.
- Study Design: Researchers might determine that only studies that used a particular research design, such as a randomized controlled trial, will be included in the review.
- Control Group: In a systematic review of an intervention, researchers might choose to include only studies that included certain types of control groups, such as a waiting list control or another type of intervention.
- Publication status : Decide whether only published studies will be included or if unpublished works, such as dissertations or conference proceedings, will also be considered.
Studies that met the following criteria were included: (a) empirical studies of couples (of any gender) who are in a committed romantic relationship, whether married or not; (b) measurement of the association between adult attachment and support in the context of this relationship; (c) the article was a full report published in English; and (d) the articles were reports of empirical studies published in peer‐reviewed journals, dissertations, review papers, and conference presentations.
Iterative Process
The iterative nature of developing a search strategy for systematic reviews stems from the need to refine and adapt the search process based on the information encountered at each stage.
A single attempt rarely yields the perfect final strategy. Instead, it is an evolving process involving a series of test searches, analysis of results, and discussions among the review team.
Here’s how the iterative process unfolds:
- Initial Strategy Formulation: Based on the research question, the team develops a preliminary search strategy, including identifying relevant keywords, synonyms, databases, and search limits.
- Test Searches and Refinement: The initial search strategy is then tested on chosen databases. The results are reviewed for relevance, and the search strategy is refined accordingly. This might involve adding or modifying keywords, adjusting Boolean operators, or reconsidering the databases used.
- Discussions and Iteration: The search results and proposed refinements are discussed within the review team. The team collaboratively decides on the best modifications to improve the search’s comprehensiveness and relevance.
- Repeating the Cycle: This cycle of test searches, analysis, discussions, and refinements is repeated until the team is satisfied with the strategy’s ability to capture all relevant studies while minimizing irrelevant results.
The iterative nature of developing a search strategy is crucial for ensuring that the systematic review is comprehensive and unbiased.
By constantly refining the search strategy based on the results and feedback, researchers can be more confident that they have identified all relevant studies.
This iterative process ensures that the applied search strategy is sensitive enough to capture all relevant studies while maintaining a manageable scope.
Throughout this process, meticulous documentation of the search strategy, including any modifications, is crucial for transparency and future replication of the systematic review.
Step 4: Search the Literature
Conduct a systematic search of the literature using clearly defined search terms and databases.
Applying the search strategy involves entering the constructed search strings into the respective databases’ search interfaces. These search strings, crafted using Boolean operators, truncation symbols, wildcards, and database-specific syntax, aim to retrieve all potentially relevant studies addressing the research question.
The researcher, during this stage, interacts with the database’s features to refine the search and manage the retrieved results.
This might involve employing search filters provided by the database to focus on specific study designs, publication types, or other relevant parameters.
Applying the search strategy is not merely a mechanical process of inputting terms; it demands a thorough understanding of database functionalities and a discerning eye to adjust the search based on the nature of retrieved results.
Step 5: screening and selecting research articles
Once the search strategy is finalized, it is applied to the selected databases, yielding a set of search results.
These search results are then screened against pre-defined inclusion criteria to determine their eligibility for inclusion in the review.
The goal is to identify studies that are both relevant to the research question and of sufficient quality to contribute to a meaningful synthesis.
Studies meeting the inclusion criteria are usually saved into electronic databases, such as Endnote or Mendeley , and include title, authors, date and publication journal along with an abstract (if available).
Study Selection
Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. PRISMA 2020 Checklist
The selection process in a systematic review involves multiple reviewers to ensure rigor and reliability.
To minimize bias and enhance the reliability of the study selection process, it is recommended that at least two reviewers independently assess the eligibility of each study. This independent assessment helps reduce the impact of individual biases or errors in judgment.
- Initial screening of titles and abstracts: After applying a strategy to search the literature, the next step involves screening the titles and abstracts of the identified articles against the predefined inclusion and exclusion criteria. During this initial screening, reviewers aim to identify potentially relevant studies while excluding those clearly outside the scope of the review. It is crucial to prioritize over-inclusion at this stage, meaning that reviewers should err on the side of keeping studies even if there is uncertainty about their relevance. This cautious approach helps minimize the risk of inadvertently excluding potentially valuable studies.
- Retrieving and assessing full texts: For studies which a definitive decision cannot be made based on the title and abstract alone, reviewers need to obtain the full text of the articles for a comprehensive assessment against the predefined inclusion and exclusion criteria. This stage involves meticulously reviewing the full text of each potentially relevant study to determine its eligibility definitively.
- Resolution of disagreements : In cases of disagreement between reviewers regarding a study’s eligibility, a predefined strategy involving consensus-building discussions or arbitration by a third reviewer should be in place to reach a final decision. This collaborative approach ensures a fair and impartial selection process, further strengthening the review’s reliability.
First, the search results from separate databases were combined, and any duplicates were removed. The lead author (S. M.) and a postgraduate researcher (F. N.) applied the described inclusion criteria in a standardized manner. First, both the titles and abstracts of the articles were evaluated for relevance. If, on the basis of the title and/or abstract, the study looked likely to meet inclusion criteria hard copies of the manuscripts were obtained. If there was doubt about the suitability of an article, then the manuscript was included in the next step. The remaining articles were obtained for full‐text review, and the method and results sections were read to examine whether the article fitted the inclusion criteria. If there was doubt about the suitability of the manuscripts during this phase, then this article was discussed with another author (C. H.). Finally, the reference lists of the eligible articles were checked for additional relevant articles not identified during the computerized search. For the selected articles (n = 43), the results regarding the relationship between attachment and support were included in this review (see Figure 1, for PRISMA flowchart).
PRISMA Flowchart
The PRISMA flowchart is a visual representation of the study selection process within a systematic review.
The flowchart illustrates the step-by-step process of screening, filtering, and selecting studies based on predefined inclusion and exclusion criteria.
The flowchart visually depicts the following stages:
- Identification: The initial number of titles and abstracts identified through database searches.
- Screening: The screening process, based on titles and abstracts.
- Eligibility: Full-text copies of the remaining records are retrieved and assessed for eligibility.
- Inclusion: Applying the predefined inclusion criteria resulted in the inclusion of publications that met all the criteria for the review.
- Exclusion: The flowchart details the reasons for excluding the remaining records.
This systematic and transparent approach, as visualized in the PRISMA flowchart, ensures a robust and unbiased selection process, enhancing the reliability of the systematic review’s findings.
The flowchart serves as a visual record of the decisions made during the study selection process, allowing readers to assess the rigor and comprehensiveness of the review.
- How to fill a PRISMA flow diagram

Step 6: Criticallay Appraising the Quality of Included Studies
Quality assessment provides a measure of the strength of the evidence presented in a review.
High-quality studies with rigorous methodologies contribute to a more robust and reliable evidence base, increasing confidence in the review’s conclusions.
Conversely, including low-quality studies with methodological weaknesses can undermine the review’s findings and potentially lead to inaccurate recommendations.
To judge the quality of studies included in a systematic review, standardized instruments, such as checklists and scales, are commonly used. These tools help to ensure a transparent and reproducible assessment process.
The choice of tool should be justified and aligned with the study design and the level of detail required. Using quality scores alone is discouraged; instead, individual aspects of methodological quality should be considered.
Here are some specific tools mentioned in the sources:
- Jadad score
- Cochrane Risk of Bias tool
- Cochrane Effective Practice and Organisation of Care (EPOC) Group Risk of Bias Tool
- Quality Assessment of Diagnostic Accuracy Studies (QUADAS)
- Newcastle – Ottawa Quality Assessment Scale for case-control and cohort studies
- EPHPP Assessment Tool
- Critical Appraisal Skills Programme (CASP) Appraisal Checklist
- Cochrane Public Health Group (CPHG)
The quality of the study was not an inclusion criterion; however, a study quality check was carried out. Two independent reviewers (S. M. and C. H.) rated studies that met the inclusion criteria to determine the strength of the evidence. The Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies was adapted to assess the methodological quality of each study (Thomas, Ciliska, Dobbins, & Micucci, 2004). The tool was adjusted to include domains relevant to the method of each study. For example, blinding was removed for nonexperimental studies. Following recommendations by Thomas et al. (2004) each domain was rated as either weak (3 points), moderate (2 points), or strong (1 point). The mean score across questions was used as an indicator of overall quality, and studies were assigned an overall quality rating of strong (1.00–1.50), moderate (1.51–2.50),
Evidence Tables
Aspects of the appraisal of studies included in the review should be recorded as evidence tables (NICE 2009): simple text tables where the design and scope of studies are summarised.
The reader of the review can use the evidence tables to check the details, and assess the credibility and generalisability of findings, of particular studies.
Critical appraisal of the quality of included studies may be combined with data extraction tables.

Step 7: extracting data from studies
To effectively extract data from studies that meet your systematic review’s inclusion criteria, you should follow a structured process that ensures accuracy, consistency, and minimizes bias.
1. Develop a data extraction form:
- Design a standardized form (paper or electronic) to guide the data extraction process : This form should be tailored to your specific review question and the types of studies included.
- Pilot test the form : Test the form on a small sample of included studies (e.g., 3-5). Assess for clarity, completeness, and usability. Refine the form based on feedback and initial experiences.
- Reliability : Ensure all team members understand how to use the form consistently.
2. Extract the data:
- General Information: This includes basic bibliographic details (journal, title, author, volume, page numbers), study objective as stated by the authors, study design, and funding source.
- Study Characteristics: Capture details about the study population (demographics, inclusion/exclusion criteria, recruitment procedures), interventions (description, delivery methods), and comparators (description if applicable).
- Outcome Data: Record the results of the intervention and how they were measured, including specific statistics used. Clearly define all outcomes for which data are being extracted.
- Risk of Bias Assessment: Document the methods used to assess the quality of the included studies and any potential sources of bias. This might involve using standardized checklists or scales.
- Additional Information: Depending on your review, you may need to extract data on other variables like adverse effects, economic evaluations, or specific methodological details.
3. Dual independent review:
- Ensure that at least two reviewers independently extract data from each study using the standardized form. Cross-check extracted data for accuracy to minimize bias and helps identify any discrepancies.
- Have a predefined strategy for resolving disagreements: This might involve discussion, consensus, or arbitration by a third reviewer.
- Record the reasons for excluding any studies during the data extraction phase. :This enhances the transparency and reproducibility of your review.
- If necessary, contact study authors to obtain missing or clarify unclear information : This is particularly important for data critical to your review’s outcomes.
- Clearly document your entire data extraction process, including any challenges encountered and decisions made. This enhances the transparency and rigor of your systematic review.
By following these steps, you can effectively extract data from studies that meet your inclusion criteria, forming a solid foundation for the analysis and synthesis phases of your systematic review.
Step 8: synthesize the extracted data
The key element of a systematic review is the synthesis: that is the process that brings together the findings from the set of included studies in order to draw conclusions based on the body of evidence.
Data synthesis in a systematic review involves collating, combining, and summarizing findings from the included studies.
This process aims to provide a reliable and comprehensive answer to the review question by considering the strength of the evidence, examining the consistency of observed effects, and investigating any inconsistencies.
The data synthesis will be presented in the results section of the systematic review.
- Develop a clear text narrative that explains the key findings
- Use a logical heading structure to guide readers through your results synthesis
- Ensure your text narrative addresses the review’s research questions
- Use tables to summarise findings (can be same table as data extraction)
Identifying patterns, trends, and differences across studies
Narrative synthesis uses a textual approach to analyze relationships within and between studies to provide an overall assessment of the evidence’s robustness. All systematic reviews should incorporate elements of narrative synthesis, such as tables and text.

Remember, the goal of a narrative synthesis is to go beyond simply summarizing individual studies. You’re aiming to create a new understanding by integrating and interpreting the available evidence in a systematic and transparent way.
Organize your data:
- Group studies by themes, interventions, or outcomes
- Create summary tables to display key information across studies
- Use visual aids like concept maps to show relationships between studies
Describe the studies:
- Summarize the characteristics of included studies (e.g., designs, sample sizes, settings)
- Highlight similarities and differences across studies
- Discuss the overall quality of the evidence
Develop a preliminary synthesis:
- Start by describing the results of individual studies
- Group similar findings together
- Identify overarching themes or trends
Explore relationships:
- Look for patterns in the data
- Identify factors that might explain differences in results across studies
- Consider how study characteristics relate to outcomes
Address contradictions:
- Consider differences in study populations, interventions, or contexts
- Look at methodological differences that might explain discrepancies
- Consider the implications of inconsistent results
- Don’t ignore conflicting findings
- Discuss possible reasons for contradictions
Avoid vote counting:
- Don’t simply tally positive versus negative results
- Instead, consider the strength and quality of evidence for each finding
Assess the robustness of the synthesis:
- Reflect on the strength of evidence for each finding
- Consider how gaps or limitations in the primary studies affect your conclusions
- Discuss any potential biases in the synthesis process
Step 9: discussion section and conclusion
Summarize key findings:.
- Summarize key findings in relation to your research questions
- Highlight main themes or patterns across studies
- Explain the nuances and complexities in the evidence
- Discuss the overall strength and consistency of the evidence
- This provides a clear takeaway message for readers
Consider study quality and context:
- Assess whether higher quality studies tend to show different results
- Examine if findings differ based on study setting or participant characteristics
- This helps readers weigh the relative importance of conflicting findings
Discuss implications:
- For practice: How might professionals apply these findings?
- For policy: What policy changes might be supported by the evidence?
- Consider both positive and negative implications
- This helps translate your findings into real-world applications
Identify gaps and future research:
- Point out areas where evidence is lacking or inconsistent
- Suggest specific research questions or study designs to address these gaps
- This helps guide future research efforts in the field
State strengths and limitations:
- Discuss the strengths of your review (e.g., comprehensive search, rigorous methodology)
- Acknowledge limitations (e.g., language restrictions, potential for publication bias)
- This balanced approach demonstrates critical thinking and helps readers interpret your findings
Minimizing Bias
To reduce bias in a systematic review, it is crucial to establish a systematic and transparent review process that minimizes bias at every stage. Sources provide insights into strategies and methods to achieve this goal.
- Protocol development and publication: Developing a comprehensive protocol before starting the review is essential. Publishing the protocol in repositories like PROSPERO or Cochrane Library promotes transparency and helps avoid deviations from the planned approach, thereby minimizing the risk of bias.
- Transparent reporting: Adhering to reporting guidelines, such as PRISMA, ensures that all essential aspects of the review are adequately documented, increasing the reader’s confidence in the transparency and completeness of systematic review reporting.
- Dual independent review: Employing two or more reviewers independently at multiple stages of the review process (study selection, data extraction, quality assessment) minimizes bias. Any disagreements between reviewers should be resolved through discussion or by consulting a third reviewer. This approach reduces the impact of individual reviewers’ subjective interpretations or errors.
- Rigorous quality assessment: Assessing the methodological quality of included studies is crucial for minimizing bias in the review findings. Using standardized critical appraisal tools and checklists helps identify potential biases within individual studies, such as selection bias, performance bias, attrition bias, and detection bias.
- Searching beyond published literature: Explore sources of “grey literature” such as conference proceedings, unpublished reports, theses, and ongoing clinical trial databases.
- Contacting experts in the field : Researchers can reach out to authors and investigators to inquire about unpublished or ongoing studies
- Considering language bias : Expanding the search to include studies published in languages other than English can help reduce language bias, although this may increase the complexity and cost of the review.
Reading List
- Galante, J., Galante, I., Bekkers, M. J., & Gallacher, J. (2014). Effect of kindness-based meditation on health and well-being: a systematic review and meta-analysis . Journal of consulting and clinical psychology , 82 (6), 1101.
- Schneider, M., & Preckel, F. (2017). Variables associated with achievement in higher education: A systematic review of meta-analyses . Psychological bulletin , 143 (6), 565.
- Murray, J., Farrington, D. P., & Sekol, I. (2012). Children’s antisocial behavior, mental health, drug use, and educational performance after parental incarceration: a systematic review and meta-analysis . Psychological bulletin , 138 (2), 175.
- Roberts, B. W., Luo, J., Briley, D. A., Chow, P. I., Su, R., & Hill, P. L. (2017). A systematic review of personality trait change through intervention . Psychological bulletin , 143 (2), 117.
- Chu, C., Buchman-Schmitt, J. M., Stanley, I. H., Hom, M. A., Tucker, R. P., Hagan, C. R., … & Joiner Jr, T. E. (2017). The interpersonal theory of suicide: A systematic review and meta-analysis of a decade of cross-national research. Psychological bulletin , 143 (12), 1313.
- McLeod, S., Berry, K., Hodgson, C., & Wearden, A. (2020). Attachment and social support in romantic dyads: A systematic review . Journal of clinical psychology , 76 (1), 59-101.

IMAGES
VIDEO
COMMENTS
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer.
A Systematic Review (SR) is a synthesis of evidence that is identified and critically appraised to understand a specific topic. SRs are more comprehensive than a Literature Review, which most academics will be familiar with, as they follow a methodical process to identify and analyse existing literature (Cochrane, 2022).
This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information. We outline core standards and principles and describe commonly encountered problems.
Systematic reviews (especially when including meta-analytic pooling of quantitative data) have several unique strengths [1,5]. Specifically, they exploit systematic literature searches enabling the retrieval of the whole body of evidence pertaining to a specific clinical question.
As the lead on a systematic review project, you will need to assemble a team of people around you that can provide methodological and topic support for your review. The size of the team around you will depend on the complexity of the question and potential volume of research that will need to be screened, quality assessed and synthesised.
A systematic review is best deployed to test a specific hypothesis about a healthcare or public health intervention or exposure. By focusing on a single intervention or a few specific interventions for a particular condition, the investigator can ensure a manageable results set.
In this article, we describe the systematic review, a type of secondary research design that is used to summarize the results of prior primary research studies. Systematic reviews are considered the highest level of evidence for a particular research question.
A systematic review is a type of study that synthesises research that has been conducted on a particular topic. Systematic reviews are considered to provide the highest level of evidence on the hierarchy of evidence pyramid. Systematic reviews are conducted following rigorous research methodology.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesise all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer.
Step 2: formulate a research question. Developing a focused research question is crucial for a systematic review, as it underpins every stage of the review process. The question defines the review’s nature and scope, guides the identification of relevant studies, and shapes the data extraction and synthesis processes.